A kind of preparation method and application of polyporphyrin compound
A compound, polyporphyrin technology, applied in the field of preparation of polyporphyrin compounds, can solve the problem of low drug loading, achieve the effect of improving phototoxicity and singlet oxygen yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1: the synthesis of polyporphyrin photosensitizer (pPS)
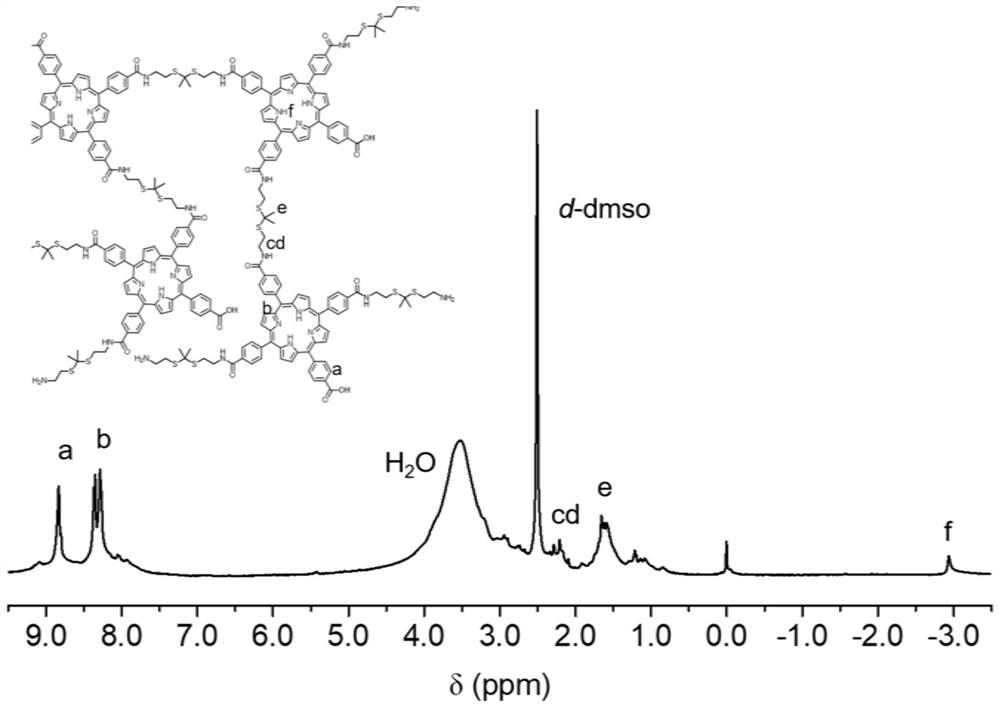
[0027] Dissolve tetraphenylporphyrin modified with four functional groups in tetrahydrofuran (0.5M, 1.0eq), add thioketal-containing comonomer with 2-terminal difunctional group of raw material into the reaction system (2.0eq), add 1-ethane Base-(3-dimethylaminopropyl)carbodiimide hydrochloride (4.8eq) and 4-dimethylaminopyridine (0.8eq), warm up to 50°C, react for 24h, and wait for the reaction bottle to cool to room temperature Carry out settling centrifugation 3 times with ether afterward, crude product is in dialysis bag (molecular weight cut-off 500) with deionization dialysis, lyophilization, the polymerization product pPS that obtains, group 1,2,3 are respectively the mol ratio of carboxylic acid / amino group is 0.8, 1, and 1.2, the molecular weight and distribution are shown in Table 1, and the NMR characterization is as follows figure 1 shown.
[0028] Table 1. Molecular weight and zeta poten...
Embodiment 2
[0030] Embodiment 2: the photodegradation experiment of polyporphyrin photosensitizer (pPS):
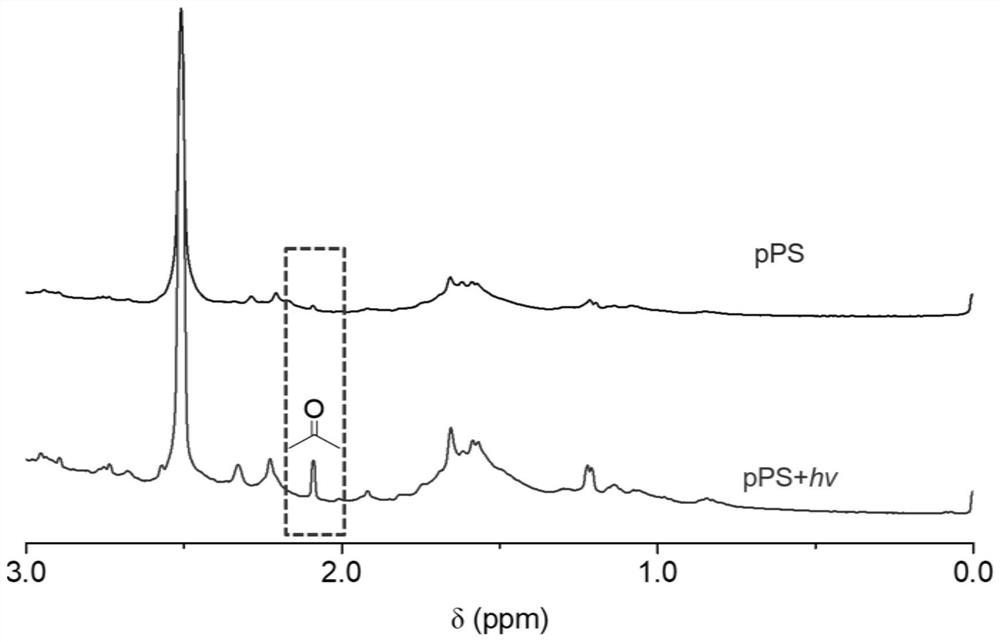
[0031] Polymer pPS (20mg) was dissolved in d-DMSO, using a 650nm laser (140mW / cm 2 ) light for 20 minutes, judged by the acetone peak at 2.1ppm in NMR that photodegradation of pPS has taken place, such as figure 2 shown.
Embodiment 3
[0032] Embodiment 3: Preparation of polyporphyrin photosensitizer nanomaterials (PEG-pPS and HA-pPS):
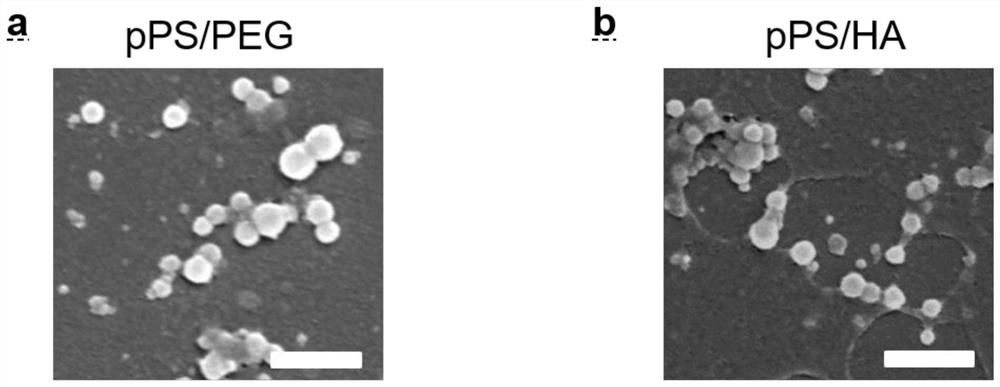
[0033] The polymer pPS was dissolved in deionized water (1 mg / mL), and PEG-COOH (1 mg / mL) and HA (1 mg / mL) were added to the pPS aqueous solution in various ratios (10 / 1-1 / 5) middle. The mixture was mixed at 37°C for 1 hour, and its particle size and shape were observed by scanning electron microscopy, as shown in image 3 As shown, the particle diameters of the two kinds of particles are both 100-200nm, and the morphology is spherical. Subsequently, the particle size changes in PBS buffer (pH 5.8, 6.8 and 8.0) of different pH values were measured by dynamic light scattering, as Figure 4 As shown in a, the particles have good stability and the particle size changes little. Subsequently, the particles were diluted in different times (1-10 times), and the particle size change was measured, such as Figure 4 As shown in b, the results show that the particle stability is ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com