Surface covalent grafting modified hexagonal boron nitride nano-sheets and preparation method thereof
A technology of hexagonal boron nitride and graft modification, which is applied in the field of surface covalent graft modification of hexagonal boron nitride nanosheets and its preparation, can solve the problem that the coupling agent and the polymer matrix are not easy to form chemical bonds, and BNNSs cannot Long-term and stable existence and other problems, to achieve the effect of low cost, improved compatibility and interfacial interaction, and mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
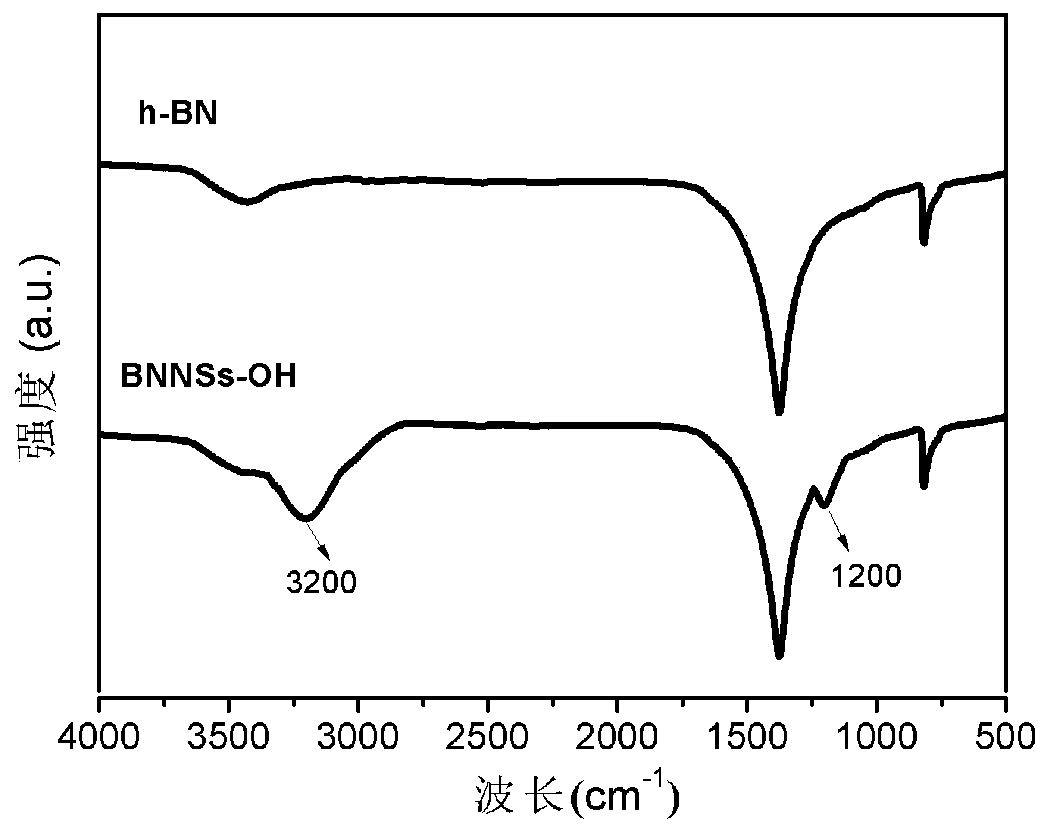
[0041] Disperse 1 g of h-BN powder in 300 ml of deionized water and keep stirring, then add 60 g of sodium hydroxide and continue stirring to obtain a mixed solution. The mixture was placed in an ultrasonic breaker, and ultrasonically peeled for 15 hours under the conditions of 80 kHz and 300 W. After ultrasonic treatment, the dispersion was centrifuged at a centrifugal speed of 3000 rpm for 30 min to remove unstripped h-BN. The supernatant was filtered and washed with deionized water until the pH of the filtrate=7. Place the filter cake at 80 o In a vacuum oven in C, 0.254 g of BNNSs-OH was obtained after drying for 8 h, with a yield of 25.4% (yield = mass of BNNSs-OH / mass of original h-BN).
[0042] figure 1The transmission electron microscope image of the BNNSs-OH prepared for BNNSs-OH shows that it is almost transparent to the electron beam, and there are obvious wrinkles and ripples, confirming its soft and thin characteristics, indicating the success of the ultra-thi...
Embodiment 2
[0044] Disperse 1 g of h-BN powder in 300 ml of deionized water and keep stirring, then add 80 g of sodium hydroxide and keep stirring to obtain a mixture. The mixture was placed in an ultrasonic breaker, and ultrasonically peeled for 15 h under the conditions of 80 kHz and 300 W. After ultrasonic treatment, the dispersion was centrifuged at a centrifugal speed of 3000 rpm for 30 min to remove unstripped h-BN. The supernatant was filtered and washed with deionized water until the pH of the filtrate=7. Place the filter cake at 80 o In a vacuum oven in C, 0.338 g of BNNSs-OH was obtained after drying for 8 h, with a yield of 33.8%.
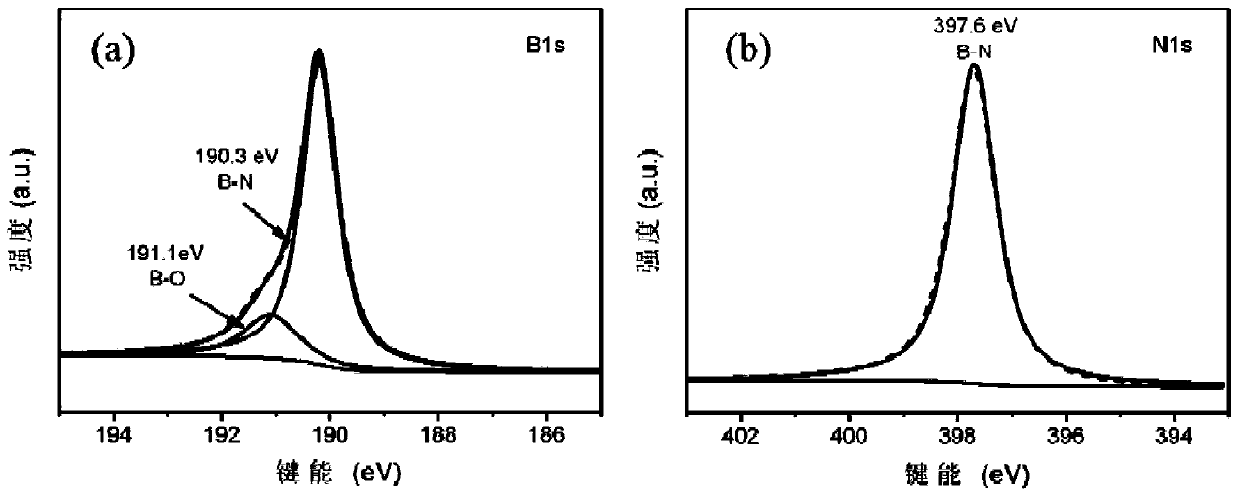
[0045] image 3 The high-resolution XPS results of B(a) and N(b) elements in the prepared BNNSs-OH show that there are B-O peaks but no N-O peaks, which means that the generated hydroxyl groups are bonded to the B atoms on the surface of BNNSs , rather than N atoms.
Embodiment 3
[0047] Disperse 1 g of h-BN powder in 300 ml of deionized water and keep stirring, then add 100 g of potassium hydroxide and keep stirring to obtain a mixture. The mixture was placed in an ultrasonic breaker, and ultrasonically peeled for 15 h under the conditions of 80 kHz and 300 W. After ultrasonic treatment, the dispersion was centrifuged at a centrifugal speed of 3000 rpm for 30 min to remove unstripped h-BN. The supernatant was filtered and washed with deionized water until the pH of the filtrate=7. Place the filter cake at 80 o In a vacuum oven in C, 0.265 g of BNNSs-OH was obtained after drying for 8 h, with a yield of 26.5%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Grafting rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com