Novel anti-tumor epirubicin compound
A compound and composition technology, applied in the field of medicine, can solve problems such as unqualified raw materials, danger to patients, and large increase in related substances, achieve good industrial value or medicinal value, reduce manufacturing costs and costs, facilitate storage and transport effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
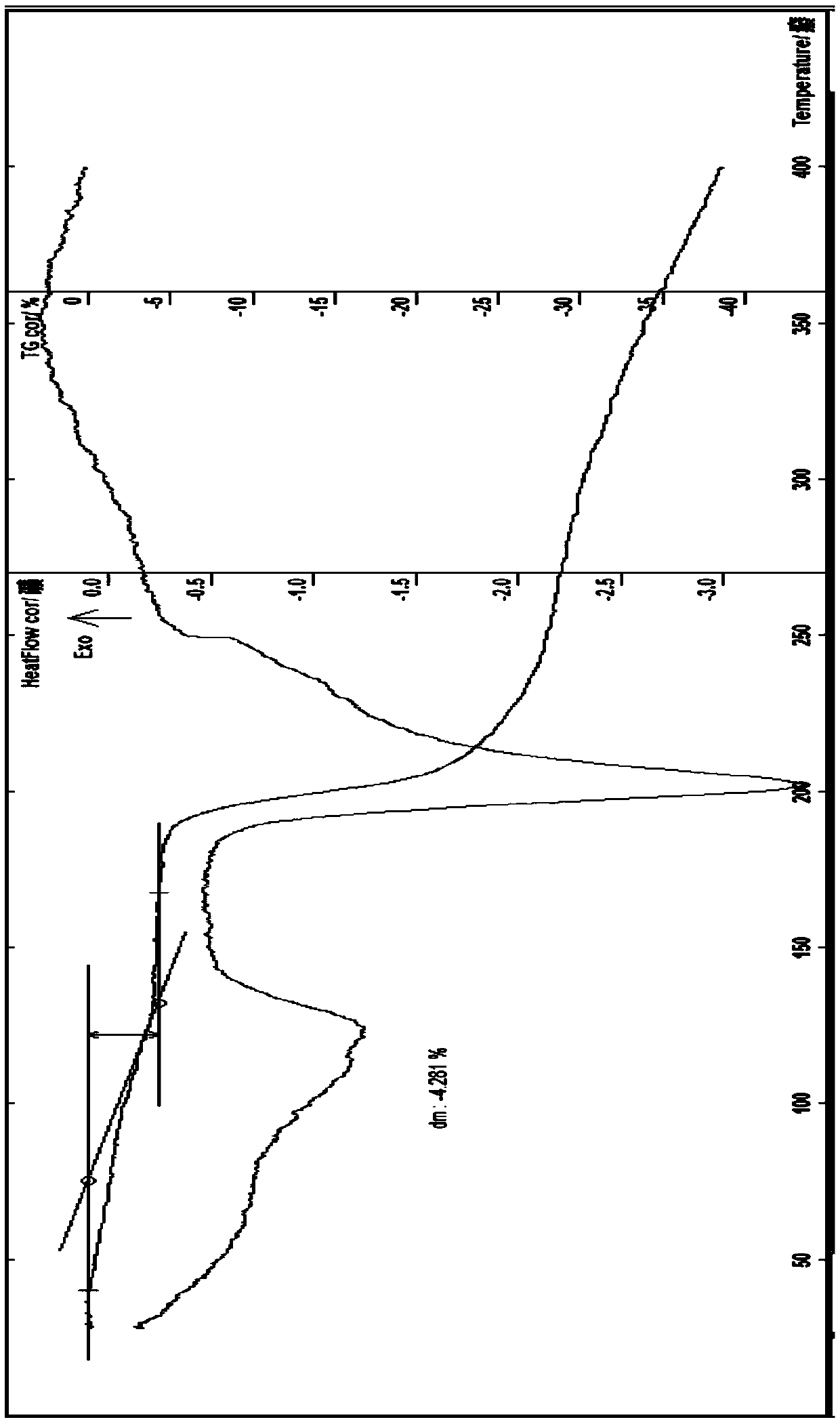
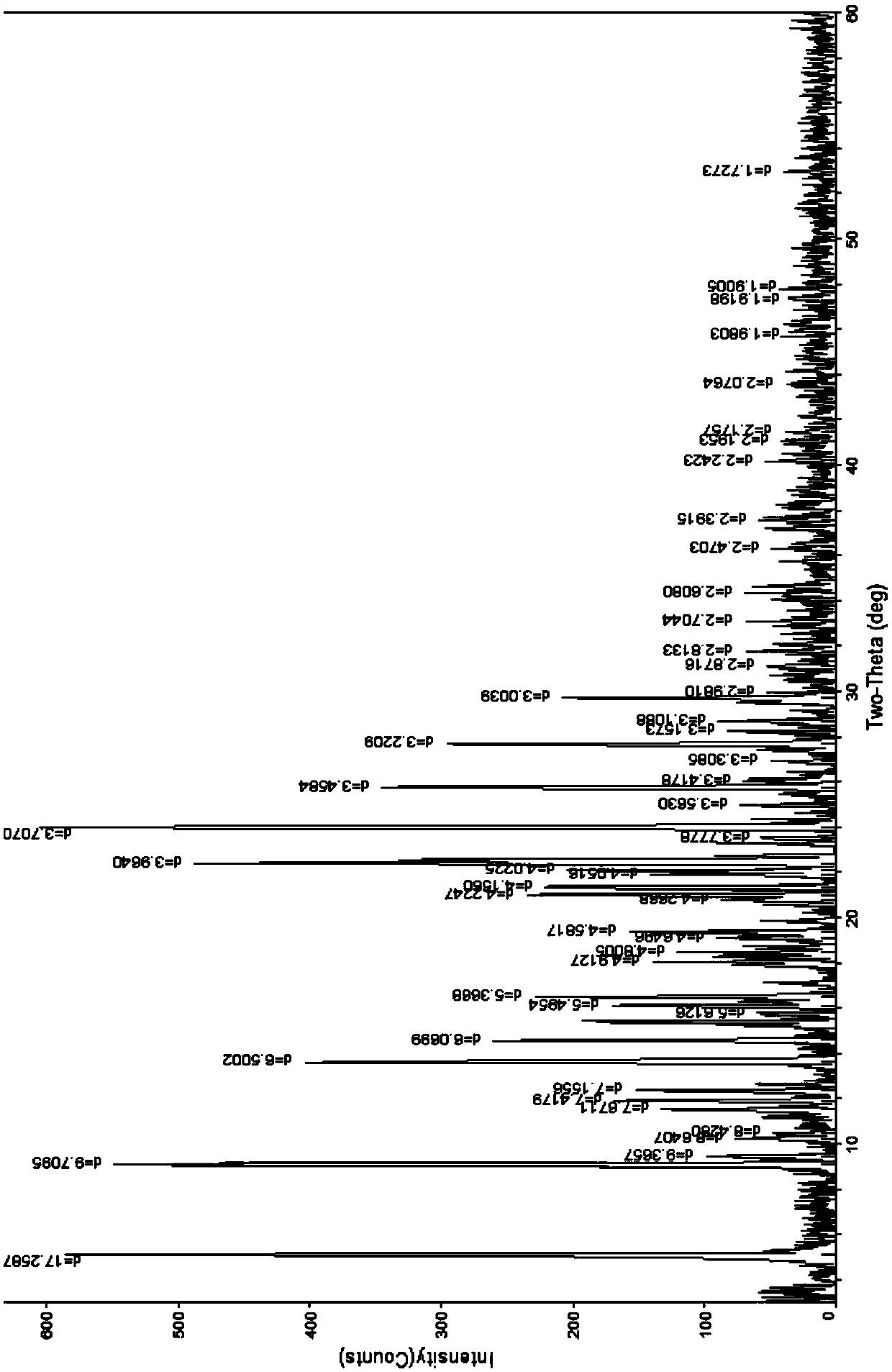
[0100] The preparation of embodiment 1 epirubicin hydrochloride 1.5 hydrate (ν type)
[0101]Add 10g of epirubicin and 20ml of dichloromethane to a 250ml three-necked flask at room temperature, pass nitrogen protection, stir, add 5% hydrochloric acid and 95% ethanol solution to adjust the pH value of the solution to about 3.4, and raise the temperature to about 30-40°C , add an appropriate amount of dichloromethane, stir to dissolve, then add 10ml of ethanol, stir, evaporate part of the solvent under reduced pressure, add 80ml of isopropanol, 5ml of ether, cool to 0°C and place it until the precipitate is fully separated, suction filtration, a small amount Wash with ether, filter with suction, dilute the obtained solid in an oven at about 40°C and blow dry for 3 hours to obtain 9.2 g of orange-red crystals; Identification: ①Take this crystal, add methanol to make a solution containing about 15 μg per 1 ml, and irradiate with ultraviolet light -Visible spectrophotometry (2010 e...
Embodiment 2
[0102] The preparation of embodiment 2 epirubicin hydrochloride 1.5 hydrate (v type compound)
[0103] Add 8g of epirubicin hydrochloride to a 250ml three-necked flask, add 40ml of 95% methanol water, protect with nitrogen, stir, stir at about 50-62°C, add an appropriate amount of methanol and stir until completely dissolved, continue stirring for 0.5 hours, Add 40ml of acetonitrile and about 50ml of isopropanol, cool to below 5°C and place it until the crystals are fully separated, then filter with suction, wash the obtained crystals twice with a small amount of ether and ethyl acetate, filter with suction, and dilute the obtained solids in an oven at 35°C. Air-dried for about 2 hours, and air-dried at about 40°C for 1 hour to obtain 7.2 g of orange-red crystalline solids; identification: ① Take this product, add methanol to make a solution containing about 20 mg per 1 ml, and use UV-visible spectrophotometry ( Appendix IV A) measured, there is maximum absorption at the wavel...
Embodiment 3
[0104] Example 3 Preparation of epirubicin hydrochloride crystalline hydrate freeze-dried powder injection (prescription: 1000 bottles)
[0105] Prescription: epirubicin hydrochloride crystalline hydrate (prepared by the method of embodiment 1 or embodiment 2, the weight of the main ingredient is calculated in terms of epirubicin hydrochloride) 10g, sorbitol 40g, disodium EDTA 0.05g, lactic acid of about 1M and Disodium hydrogen phosphate solution, appropriate amount, appropriate amount of water for injection;
[0106] Take the prescribed amount of epirubicin hydrochloride crystalline hydrate, add sorbitol, and disodium EDTA in the liquid preparation tank, add about 400ml of water for injection, stir to dissolve, and adjust the pH with about 1M lactic acid and disodium hydrogen phosphate solution to 4.5~5.5, add activated carbon 0.01% (W / V), stir for 30 minutes, filter with 0.22 micron microporous membrane, pack according to the weight of main drug 10mg / bottle, freeze at -45~-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com