Biosynthesis method for beta-alanine
A technology of alanine and acrylic acid, applied in the field of β-alanine biosynthesis, can solve problems such as low yield, complicated process, incomplete conversion, etc., shorten the reaction process, improve the preparation efficiency, and have little environmental impact Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0079] Example 1 Mutation Generation and Screening
[0080] Mutants of aspartase were prepared: T187, M321, K324, N326 and AFIL (quad mutant).
[0081] The wild-type aspartase (Gen Bank No. AB028242.1) is derived from Bacillus sp YM55-1, the nucleotide sequence is shown in SEQ ID NO: 1, the amino acid sequence is shown in SEQ ID NO: 3, Using the recombinant plasmid pET-24a (nucleotide sequence shown in SEQ ID NO: 2) containing the wild aspartase gene as a template, site-directed saturation mutations were performed at T187, M321, K324 and N326.
[0082] Use the software Oligo7 to design blunt-end primers, and the degenerate primers are as follows (wherein N=A, G, C or T; K=G or T):
[0083] Mutation of amino acid T187:
[0084] Forward primer: 5'- NNK GGTATCGGCGGTTTTG-3'
[0085] Reverse primer: 5'-ACCACCCAGACCCAGTGCCGGA-3'
[0086] Mutation of amino acid at position M321:
[0087] Forward primer: 5'- NNK CAATATATCGTAAAAGCTGCTG-3'
[0088] Reverse primer: 5'-ATGTTCTTT...
Embodiment 2
[0098] Expression of embodiment 2 aspartase mutants
[0099] Each transformant prepared in Example 1 (Escherichia coli containing mutants, and Escherichia coli expressing wild-type aspartase as a control) was respectively inoculated in 5 ml of kanamycin-containing (50 μg / ml) In LB medium, culture in a shaker at 37°C and 200rpm until OD 600 When it reaches about 0.4 to 0.5, a seed liquid is obtained.
[0100] Each seed solution was inoculated in autoinduction medium (Qingdao Haibo) at an inoculum amount of 1%, and cultured at 30° C. for 24 hours. The bacterial solution was ultrasonically disrupted, and the supernatant was obtained after centrifugation, which was the corresponding enzyme solution.
Embodiment 3
[0101] The catalysis of embodiment 3 aspartase mutants to acrylic acid
[0102]
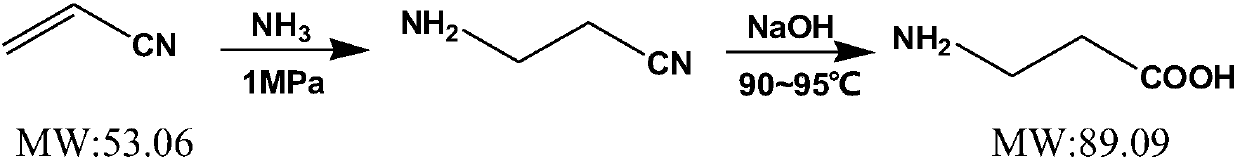
[0103] β-Alanine Synthetic Route
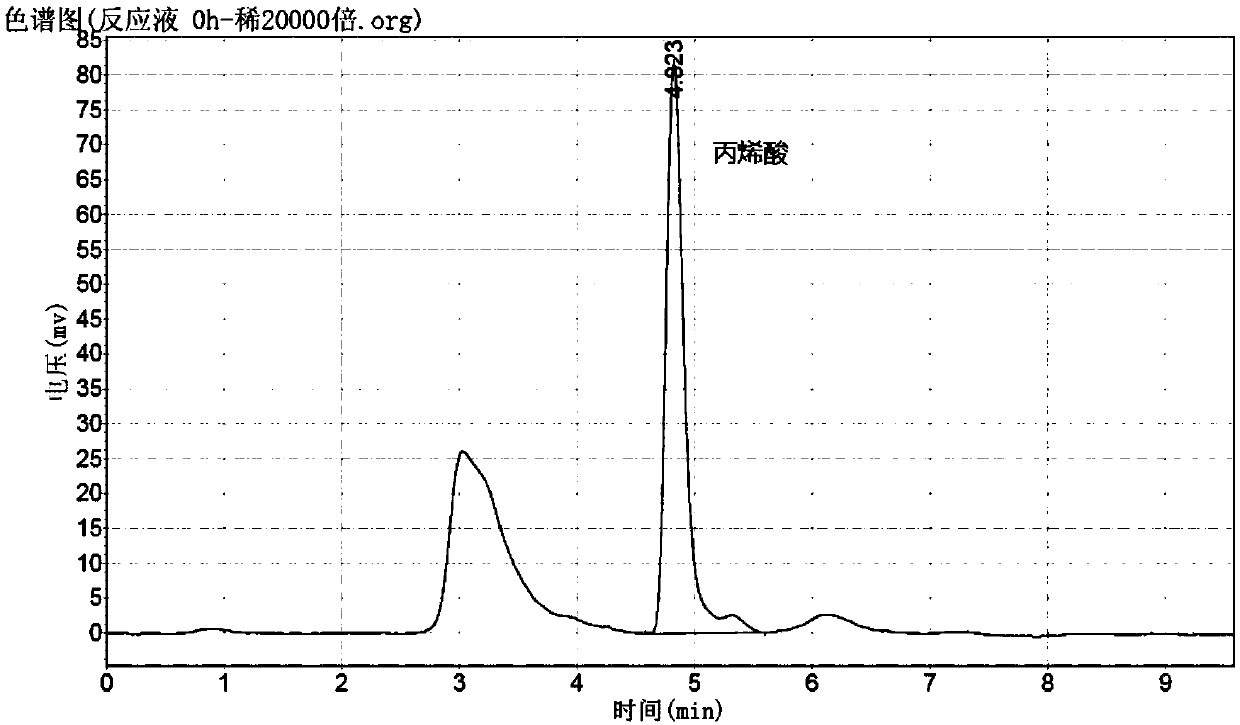
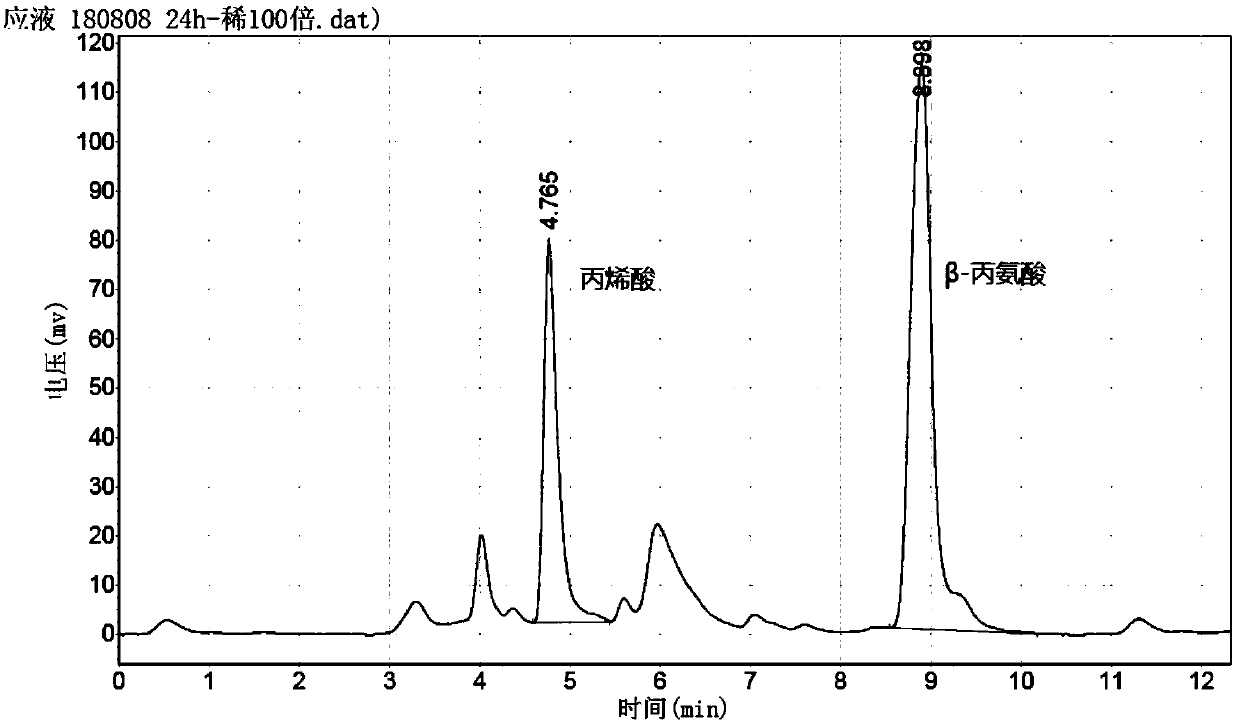
[0104] Add 23ml of 25% ammonia water to a 100ml three-necked flask, start stirring at 25rpm, slowly add 5ml of glacial acetic acid (AR), add 6.0g of acrylic acid, stir and heat in a water bath to 48-51°C, the system pH is 9.0-9.2, add MgSO 4 ·7H 2 0.13 mg, add 9 ml of the enzyme solution prepared in Example 2, retest pH 8.9-9.1, and after 24 hours of incubation at 48 to 51° C., get 100 μl of the reaction solution and dilute 1000 times with the mobile phase for HPLC liquid phase analysis.
[0105] Liquid phase conditions: C18 column (25cm), [3% NaH 2 PO 4 2H 2 O (pH3.3-3.5): water = 1: 3]: acetonitrile = 85: 15; flow rate 1ml / min, detection wavelength: 205nm.
[0106] Retention time: β-alanine: 2.66min; Acrylic acid: 5.17min
[0107] Conversion rate=[1-(residual acrylic acid content / initial acrylic acid content)]×100%
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com