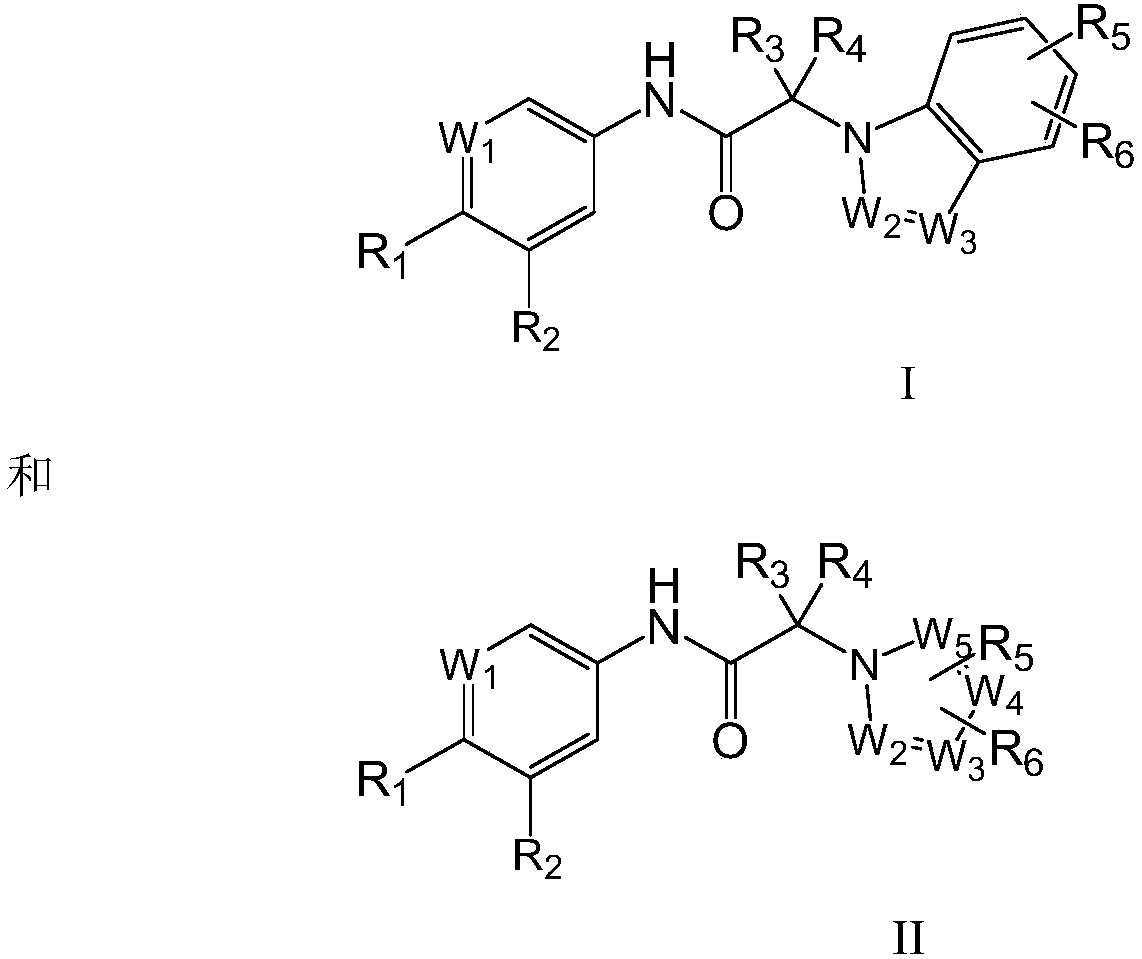
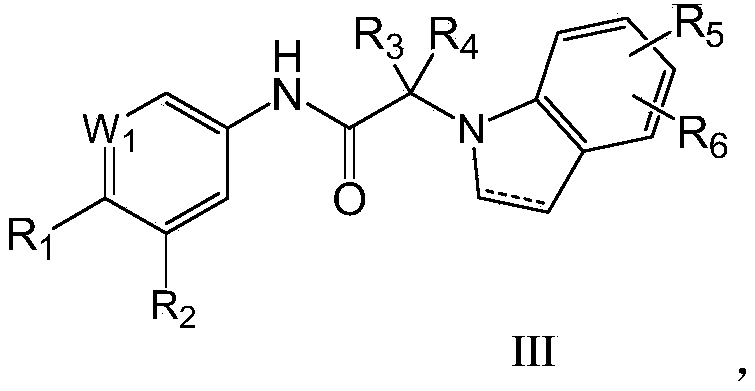
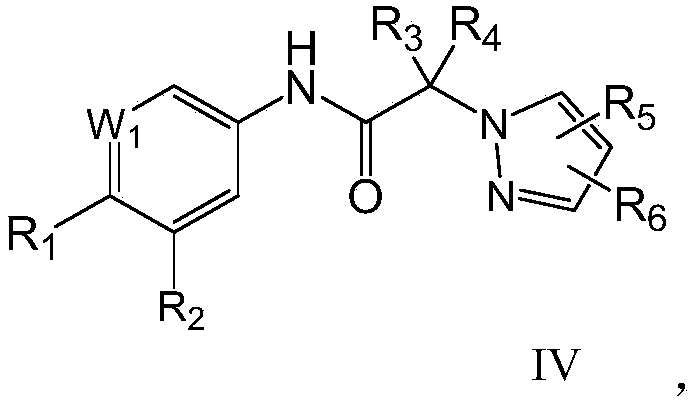
N-aromatic amide compound and preparation method and application thereof
An aromatic amide and compound technology, which is applied to N-aromatic amide compounds and the fields of their preparation and use, can solve the problems of convulsions of patients, high price, application limitation and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0086] N-(4-cyano-3-trifluoromethyl)phenyl-2-[4-fluoro-6-(4-fluorophenyl)-1H-indol-1-yl]-2-methylpropane Preparation of amides
[0087]
[0088] first step reaction
[0089] Thionyl chloride (2.62 mL, 35.93 mmol, 1.2 eq) was added dropwise to a solution of 2-bromo-2-methylpropionic acid (5.00 g, 29.94 mmol) in 30 mL of dry tetrahydrofuran (THF) , The temperature during the dropwise addition is controlled at 0-12°C, and the time is 10 minutes. The resulting mixture was stirred under the same conditions for 2 hours. The internal temperature was adjusted to about -5°C, and triethylammonia (Et 3 N) (5.42ml, 38.92mmol, 1.3eq), the internal temperature was lower than 12°C during the addition. Stir for 20 minutes under the same reaction conditions. A solution of 4-cyano-3-trifluoromethyl-aniline (5.57 g, 29.94 mmol) in 30 mL of anhydrous tetrahydrofuran was then added dropwise thereto, and the resulting mixture was stirred at 50° C. for two hours. After the thin layer chroma...
Embodiment 2
[0100] Preparation of N-(4-cyano-3-trifluoromethyl)phenyl-2-(5-fluoro-6-phenyl-1H-indol-1-yl)-2-methylpropionamide
[0101]
[0102] first step reaction
[0103] With embodiment 1.
[0104] second step reaction
[0105] 6-Bromo-5-fluoro-1H-indole (1.00 g, 4.672 mmol) was added to tetrakis(triphenylphosphine)palladium [Pd(PPh 3 ) 4 ] (0.54 g, 0.4672 mmol) in a suspension of 20 ml of ethylene glycol dimethyl ether (DME), stirred at room temperature for 15 minutes under the protection of argon. A solution of phenylboronic acid (0.57 g, 4.672 mmol) in 2.5 mL of ethanol was added to the above reaction solution, and the resulting mixture was stirred under the same conditions for 10 minutes. Potassium carbonate (0.97 g, 7.008 mmol) in 2.0 mL of water was then added to the above reaction solution. The resulting reaction mixture was heated to reflux for 2-3 hours under the protection of argon. After the reaction was confirmed by thin layer chromatography, brine (20 ml) and eth...
Embodiment 3
[0112] Preparation of N-(4-cyano-3-trifluoromethyl)phenyl-2-(5-fluoro-1H-indol-1-yl)-2-methylpropionamide
[0113]
[0114] In a 100 mL round bottom flask was added 2-bromo-N-(4-cyano-3-trifluoromethyl-phenyl)-2-methylpropanamide (0.20 g, 0.5968 mmol), 5-fluoro-1H -Indole (0.16 g, 1.1963 mmol), cuprous bromide dimethyl sulfide (12.3 mg, 0.05868 mmol), triphenylphosphine (15.7 mg, 0.05968 mmol), sodium hydroxide (23.9 mg, 0.5968 mmol), 10 mL of anhydrous toluene as solvent. The resulting mixture was heated to 50 °C and stirred under argon protection for 12 hours. After thin-layer chromatography confirmed that the reaction was complete, the reaction solution was cooled to 20 ± 5 ° C, then water (30 ml) and ethyl acetate (30 ml) were added, the liquids were separated after brief stirring, and the organic phase was washed with brine (20 ml). Dried over magnesium sulfate, filtered and the organic phase was drained to give an oily substance. Separation by silica gel column chr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Nmr spectrum | aaaaa | aaaaa |
| Nmr spectrum | aaaaa | aaaaa |
| Nmr spectrum | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com