Pyrrole-naphthalimide derivative fluorescent probe as well as preparation method and application thereof
A fluorescent probe and naphthalimide technology, applied in fluorescence/phosphorescence, chemical instruments and methods, luminescent materials, etc., can solve the problems of complex purification process, long synthesis route, high cost of raw materials, etc., and achieve synthesis and post-processing methods Simple, easy to obtain raw materials, wide application value effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] The preparation method of the pyrrole-naphthalimide derivative fluorescent probe in this embodiment is as follows:
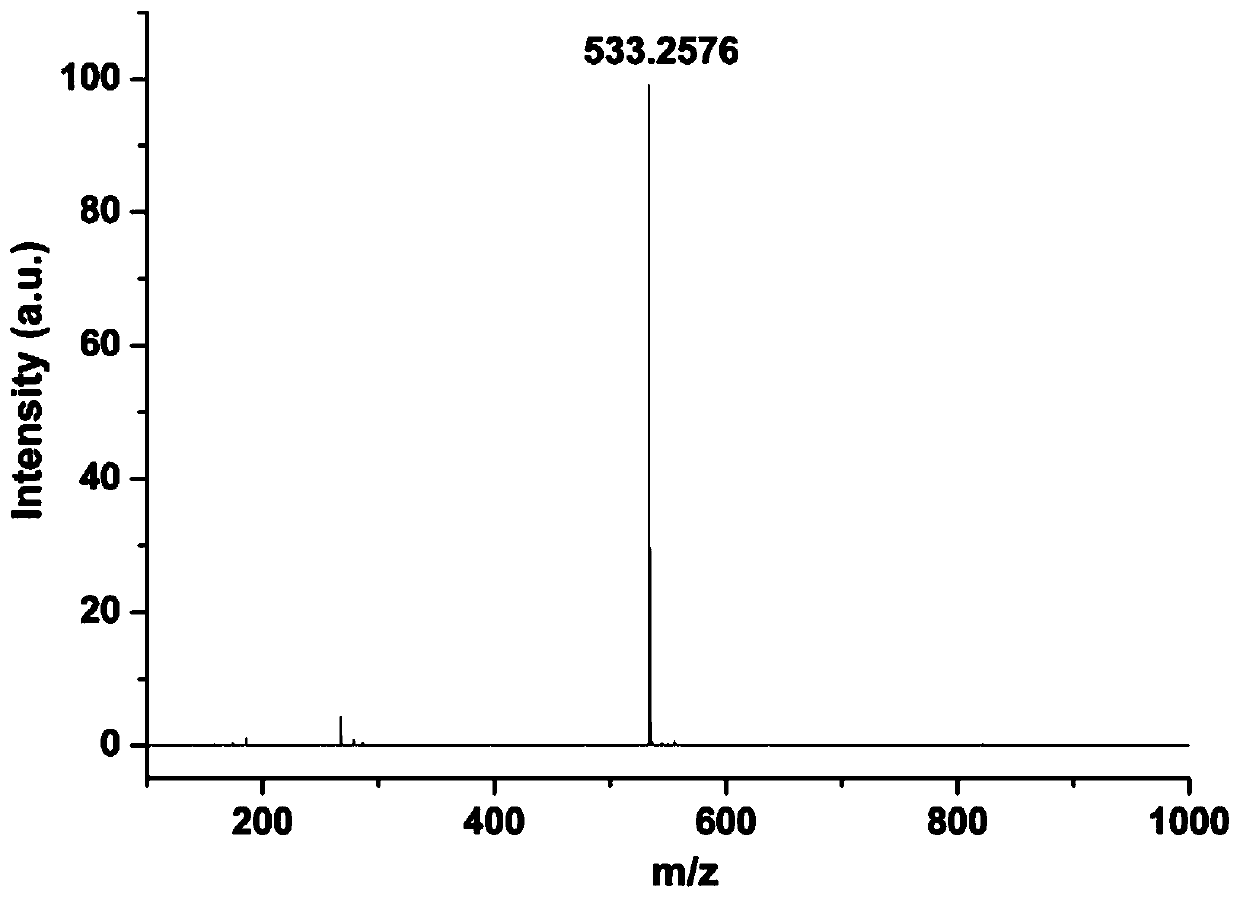
[0032] 2.79 g (10 mmol) N-morpholinoethyl-2,4-dimethyl-5-formylpyrrole-3-carboxamide and 2.71 g (10 mmol) 4-hydrazino-N-hydroxyethylnaphthoylidene Dissolve the amine in 0.05L ethanol, add dropwise 0.012g acetic acid (0.2 mmol) as a catalyst, reflux and stir at 80°C for 3-4h, cool and stand to room temperature, filter under reduced pressure, and wash the obtained solid with ethanol to obtain the pyrrole- Fluorescent probes of naphthalimide derivatives. The yield of the target product was 86%.
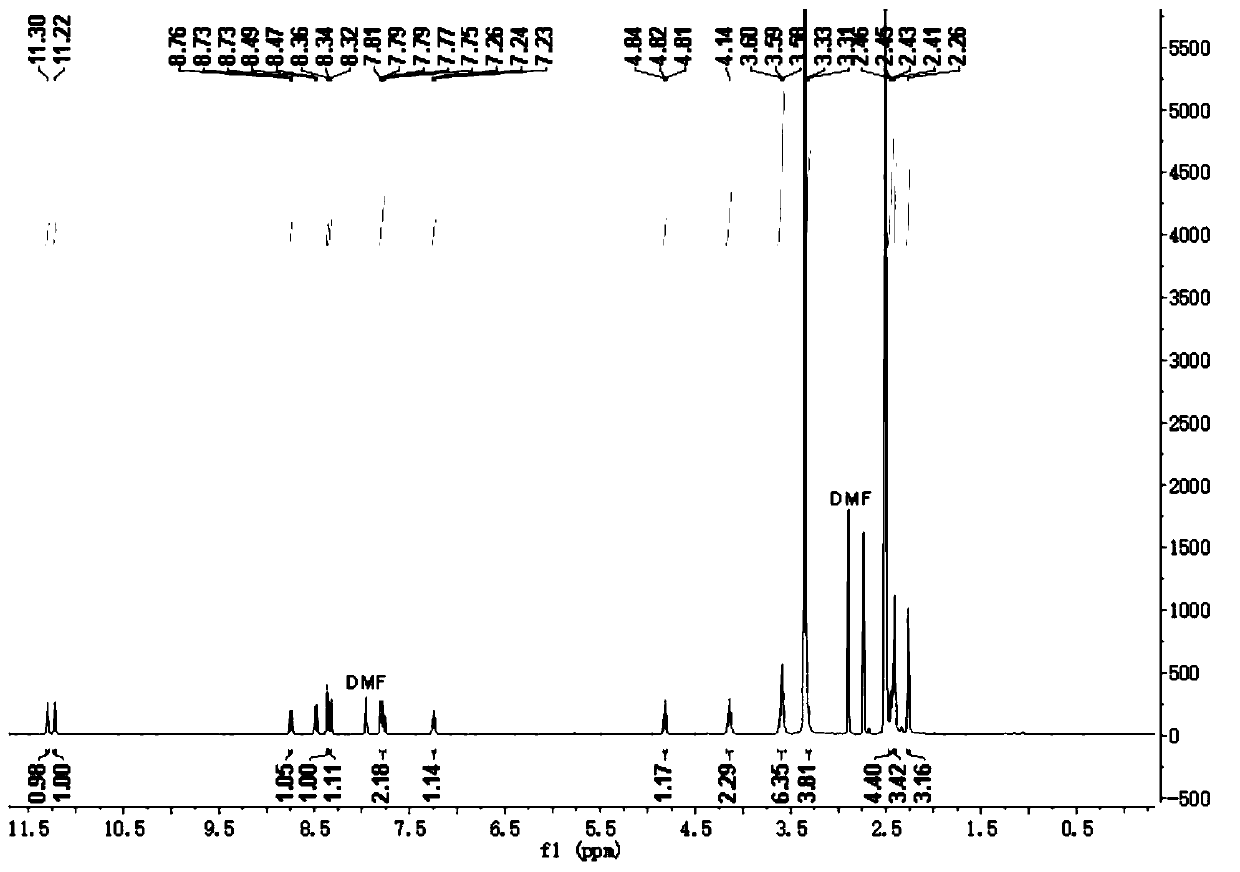
[0033] Adopt nuclear magnetic resonance instrument to carry out nuclear magnetic resonance analysis to the prepared pyrrole-containing merocyanine derivatives, the results are as follows:
[0034] 1 H NMR (400 MHz, DMSO- d 6 ), δ (ppm): 11.30 (s, 1H, NH-pyrrole), 11.22 (s,1H, NH), 8.73-8.76 (d, 1H, Aryl-H), 8.47-8.49 (d, 1H, Aryl- H), 8.36 (s, 1H, CH=N), 8.32-...
Embodiment 2
[0037] Determination of Optical Properties of Copper Ions Containing Pyrrole-Naphthimide Derivatives
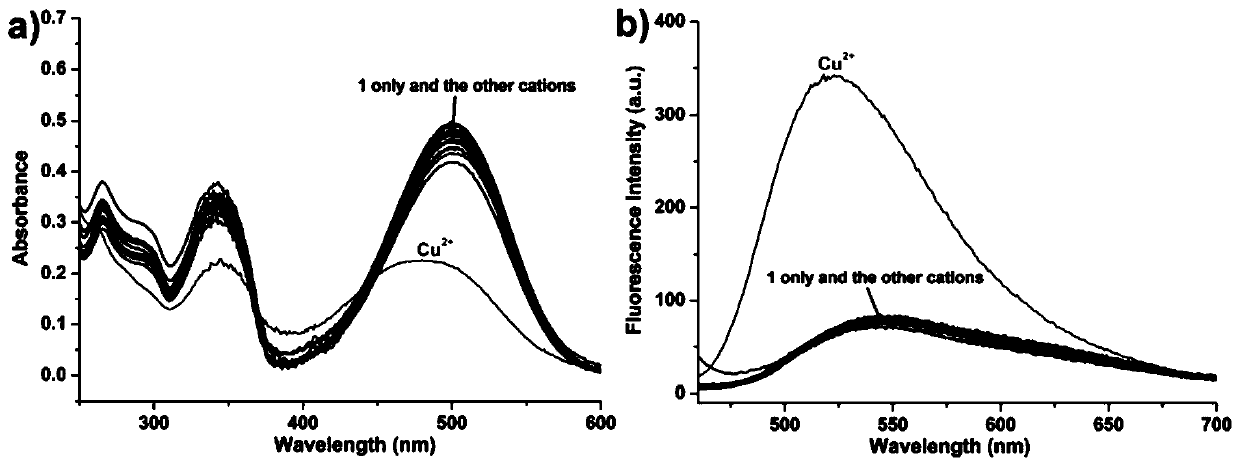
[0038] The pyrrole-naphthalimide derivatives prepared in the above example 1 were used as fluorescent probes in acetonitrile / HEPES buffer solution (v:v, 3:7, 0.02 mol / L, pH=5) to prepare a molar concentration of 1×10 -5 mol / L solution, respectively, at a molar concentration of 2×10 -5 mol / L metal ion (Ag + , Al 3+ , Ca 2+ , Cd 2+ , Co 2+ , Cr 3+ , Cu 2+ , Fe 3+ , Hg 2 + ,K + , Mg 2+ , Mn 2+ , Na + , Ni 2+ , Pb 2+ and Zn 2+ ) solution, adding an equal amount of the above-mentioned fluorescent probe solution, and using a UV-Vis spectrophotometer or a fluorescence spectrometer for analysis (excitation wavelength is 440 nm), the resulting UV and fluorescence spectra are shown in image 3 . pass image 3 It can be seen that the pyrrole-naphthoimide derivative prepared in the present invention is used as a probe to only have a significant response to copper io...
Embodiment 3
[0041] Detection experiment of pyrrole-naphthalimide derivative fluorescent probe in intracellular copper ion
[0042] 1 x 10 for HeLa cells -5 mol / L pyrrole-naphthalimide derivative fluorescent probe prepared in Example 1 above was incubated at 37°C for 30 minutes, and Cu 2+ (2×10 -5 mol / L) and then incubated for 30 minutes to obtain the fluorescence imaging image of HeLa cells, as shown in Figure 5 As shown, wherein: a is the fluorescence imaging image of the green channel of the above-mentioned fluorescent probe; b is the bright-field image of the above-mentioned fluorescent probe; c is the superimposed picture of the bright-field image and the fluorescence image of the above-mentioned fluorescent probe; d is the image of the above-mentioned fluorescent probe Needle + Cu 2+ Green channel fluorescence imaging; e is the above fluorescent probe + Cu 2+ Imaging image under bright field; f is the above-mentioned fluorescent probe Cu 2+ Superimposed images of brightfield ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com