N-ethyl pyridine methylamine hydrochloride crystal, preparation process thereof and application of N-ethyl pyridine methylamine hydrochloride crystal in preparation of tropicamide
A technology of ethyl picolinium hydrochloride and ethyl picolinium, which is applied in the field of pharmaceutical preparation, can meet the market demand of low-purity N-ethyl picolinium, the N-ethyl picolinium has a low melting point, Problems such as high viscosity of N-ethylpicolinium
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Preparation of Compound 1: Add 157.8g (1.4mol) of 4-pyridinecarbaldehyde and 2000mL of absolute ethanol to a 5000mL three-neck flask, stir at room temperature to obtain a clear solution, control the temperature in an ice-water bath at 0-10°C, and add 214.6g (1.5mol) dropwise 66% concentration of ethylamine aqueous solution, after dripping, react for 1-2 hours, control in the TLC plate, if the raw material point disappears, the reaction is considered complete, slowly add 58.5g (1.5mol) sodium borohydride in batches, after adding, control React at 10-25°C for 1-2 hours, add 300mL of purified water dropwise, stir overnight at room temperature, and control in TLC. After the reaction is complete, concentrate in vacuo to remove the solvent, add 2000mL of water, extract with 2000mL of dichloromethane, and saturate the organic phase with 1500mL Wash with brine, dry over anhydrous sodium sulfate, filter, and concentrate the filtrate in vacuo to obtain 192.5 g of dark yellow oil, ...
Embodiment 2
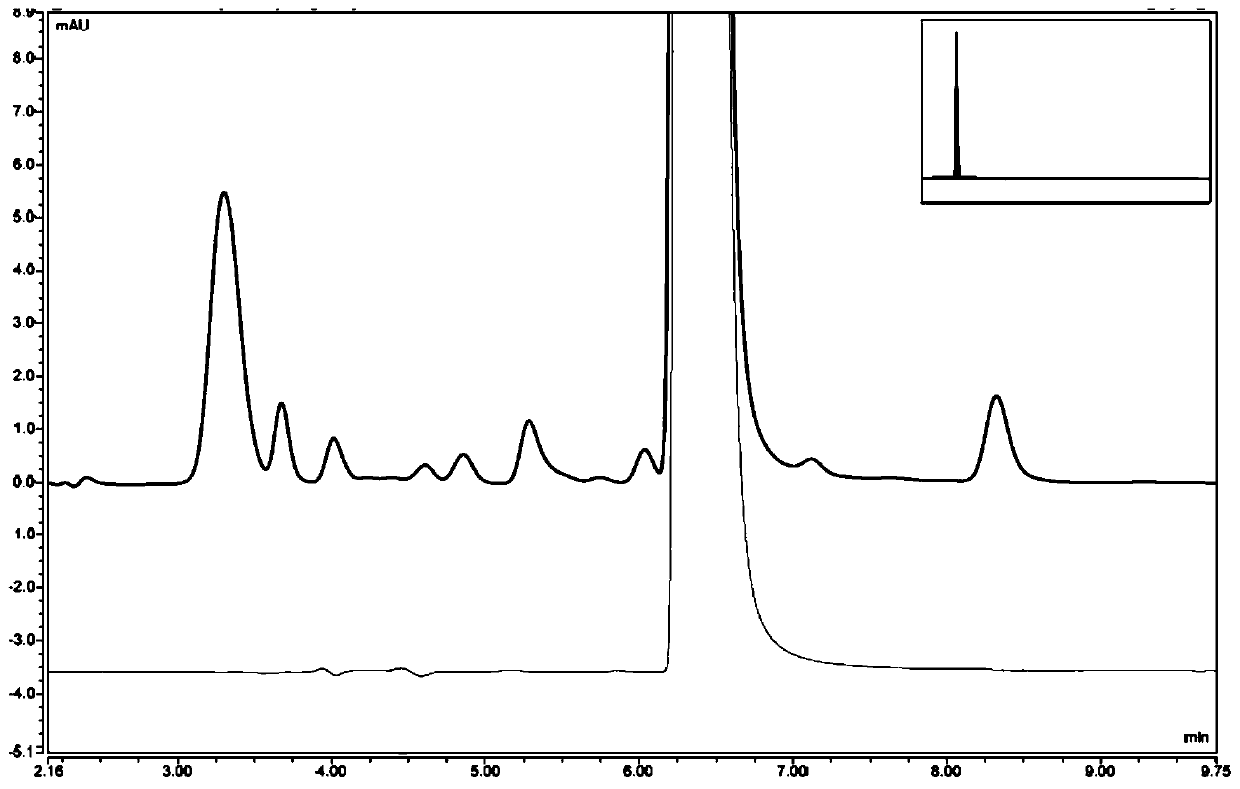
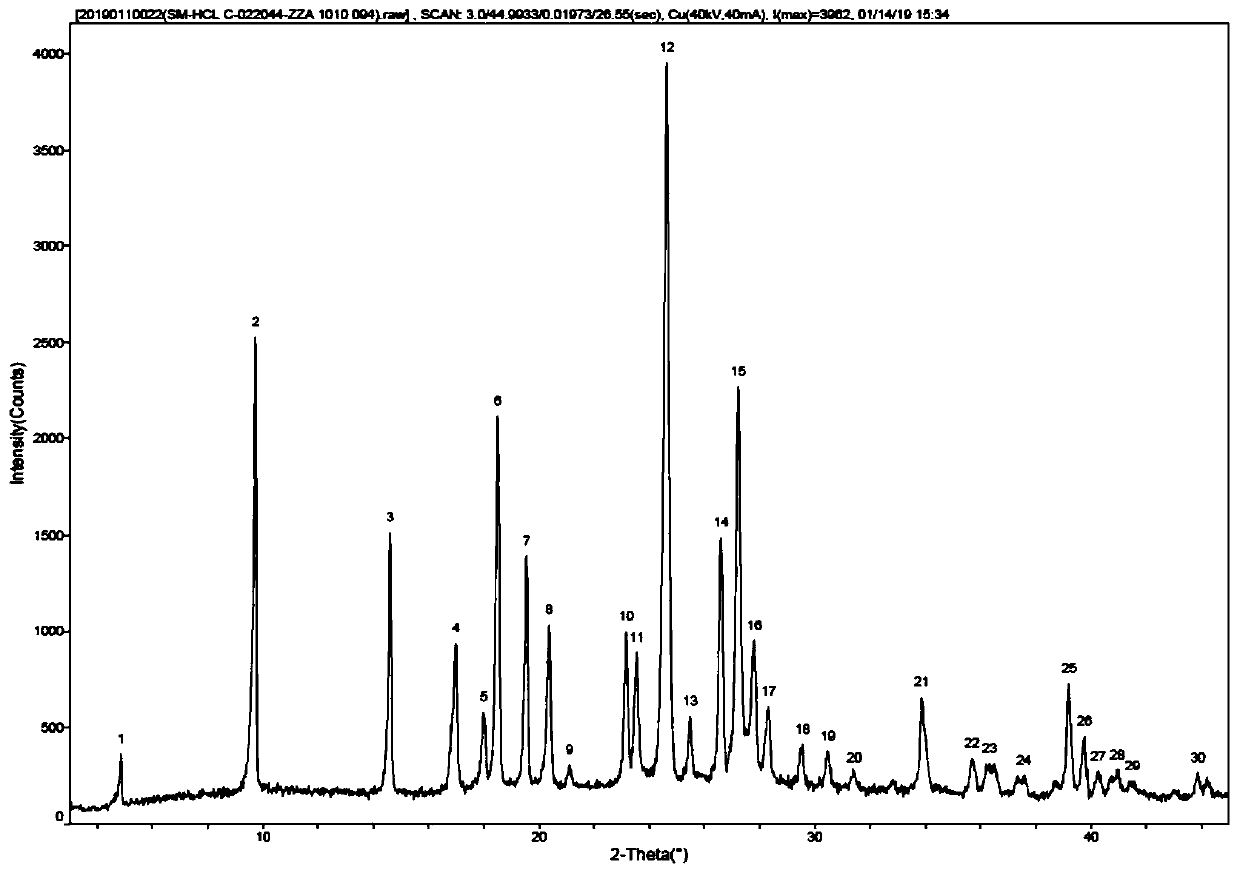
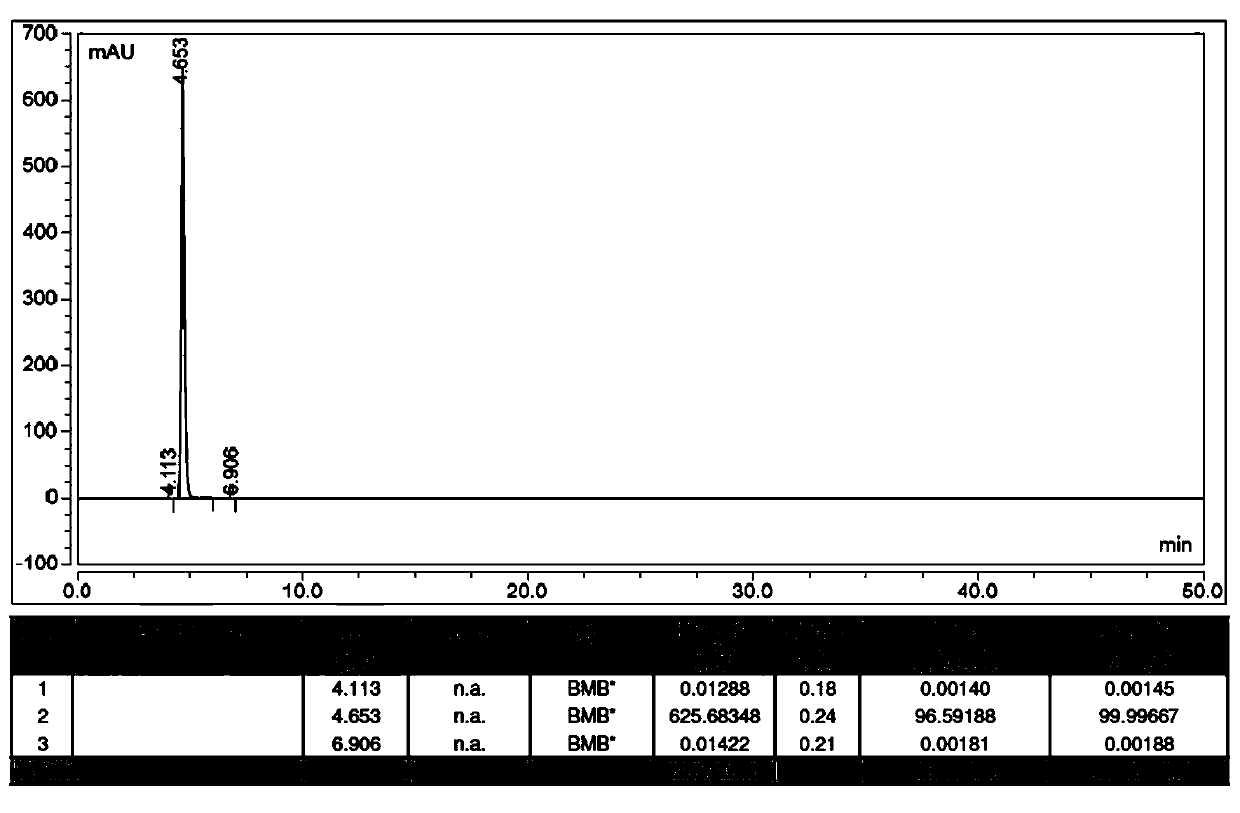
[0049] Preparation of Compound 2: Add 15.1g (0.11mol) N-ethylpicolinylamine and 50mL isopropanol to a 100mL three-neck flask, stir at room temperature to obtain a light yellow clear solution, slowly add 14.25g (0.12mol) 31% concentration hydrochloric acid, dripped, stirred at room temperature for 1-5 hours, filtered, the filter cake was washed with acetonitrile, and dried in vacuo to obtain 17.5 g of white crystals with a purity of 99.8% and a yield of 91.7%. figure 2 Shown XRD pattern, this pattern feature is shown in Table 1.
Embodiment 3
[0051] Preparation of Compound 2: Add 10.2g (0.075mol) of pure N-ethylpicolinylamine and 60mL of ethyl acetate to a 100mL three-neck flask, stir at room temperature to obtain a light yellow clear solution, slowly add 9.7g (0.08mol) of 31 % concentration of hydrochloric acid, dripped, stirred at room temperature for 1-5 hours, filtered, the filter cake was washed with acetonitrile, and vacuum-dried to obtain 11.44 g of white crystals with a purity of 99.9% and a yield of 88.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com