Thrombin-loaded microsphere-expanded sponge composite hemostatic material and preparation method and application thereof
A technology of hemostatic material and thrombin, which is applied in the field of medical devices, can solve problems such as difficulty in bonding wound surfaces, decreased hemostatic effect, and difficulty in exerting the effect of thrombin, achieving good clinical application prospects, avoiding vascular embolism, and rich sources of raw materials Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
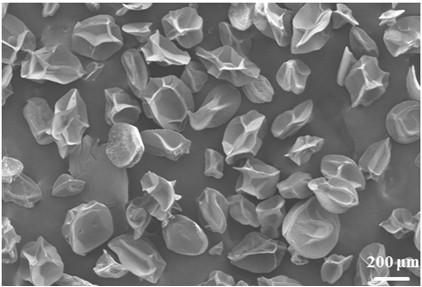
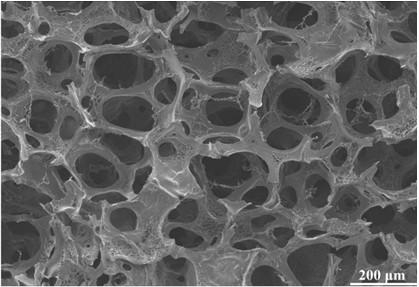
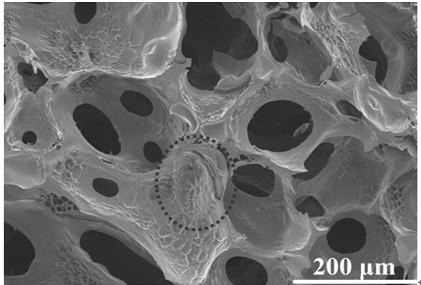
Image
Examples
Embodiment 1
[0029] A preparation method for carrying thrombin microspheres-expandable sponge composite hemostatic material, comprising the following steps:
[0030] (1) Preparation of sodium alginate-loaded thrombin microspheres, after mixing sodium alginate solution with a mass concentration of 2% and thrombin solution (enzyme activity is 20 iu / mL) for electrostatic spraying and collecting a mass concentration of 0.5 % calcium chloride solution, followed by washing with ultrapure water and freeze-drying to obtain sodium alginate-loaded thrombin microspheres. The working parameters of the electrostatic spray are: voltage 5KV, flow rate 1 mL / h, distance from needle to receiver 8cm.
[0031] (2) Using PBS buffer as a solvent, prepare a carboxymethyl chitosan grafted methacrylic acid solution with a mass concentration of 1%.
[0032] (3) Add 50% cystine and 0.05% sodium alginate-loaded thrombin microspheres to the carboxymethyl chitosan-grafted methacrylic acid solution.
[0033] (4) Add p...
Embodiment 2
[0035] A preparation method for carrying thrombin microspheres-expandable sponge composite hemostatic material, comprising the following steps:
[0036] (1) Preparation of sodium hyaluronate-loaded thrombin microspheres, mix sodium hyaluronate solution with a mass concentration of 5% and thrombin solution (enzyme activity is 50 iu / mL) for electrostatic spraying and collect the mass concentration 5% glutaraldehyde solution, followed by washing with ultrapure water and freeze-drying to obtain sodium hyaluronate-loaded thrombin microspheres. The working parameters of the electrostatic spray are: voltage 10KV, flow rate 2 mL / h, distance from needle to receiver 12cm.
[0037] (2) Using PBS buffer as a solvent, prepare a sodium alginate grafted maleimide solution with a mass concentration of 50%.
[0038] (3) Add 10% cystine and 5% sodium hyaluronate-loaded thrombin microspheres to the above-mentioned sodium alginate-grafted maleimide solution.
[0039] (4) Add photoinitiator 1173...
Embodiment 3
[0041] A preparation method for carrying thrombin microspheres-expandable sponge composite hemostatic material, comprising the following steps:
[0042] (1) Preparation of dextran-loaded thrombin microspheres. Mix 3% dextran solution with thrombin solution (enzyme activity: 100 iu / mL) for electrostatic spraying and collect a concentration of 1 % genipin solution, followed by washing with ultrapure water and freeze-drying to obtain dextran-loaded thrombin microspheres. The working parameters of the electrostatic spray are: voltage 12KV, flow rate 8 mL / h, distance from needle to receiver 15cm.
[0043](2) Using PBS buffer as a solvent, prepare a hyaluronic acid grafted isocyanate solution with a mass concentration of 10%.
[0044] (3) Add 5% mercapto-polyethylene glycol-mercapto (weight average molecular weight 2000-10000) and 5% dextran-loaded thrombin microspheres to the above hyaluronic acid-grafted isocyanate solution.
[0045] (4) Add photoinitiator 2959 with a mass conce...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com