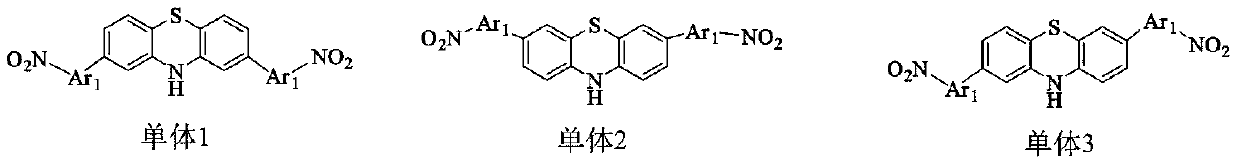
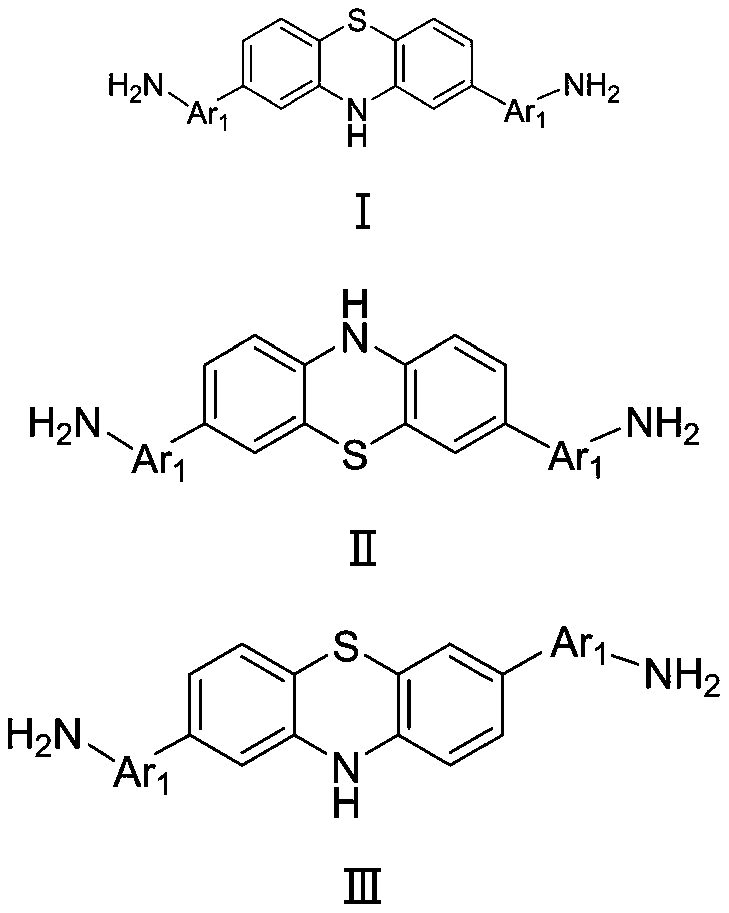
Antibacterial diamine prepared from medical intermediate and preparation method thereof
A system and medical technology, applied in the field of material science, can solve problems such as high cost and complicated preparation process, and achieve the effects of improving heat resistance, compact packing, and improving barrier performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] This example provides the synthesis of 4,4'-(10H-phenothiazine-2,8-diyl)dianiline:
[0035]
[0036] S1. Add 3.57g (0.01mol) 2,8-dibromo-10H-phenothiazine, 4.335g (0.025mol) p-aminophenyl borate hydrochloride into a 500ml three-necked flask, tetrahydrofuran (THF) as the solvent, and then add 2mol 37.5ml of potassium carbonate solution per L and an appropriate amount of Aliquat336, magnetic stirring and argon gas, after heating the oil bath to 75°C, add 0.020g tetrakistriphenylphosphine palladium, reflux reaction for 24h, add water to quench the reaction, and then use organic Solvent extraction, and then distill off the solvent under reduced pressure.
[0037] S2. The product after the solvent is evaporated in step S1 is purified by column chromatography with dichloromethane:n-hexane=1:1 (volume ratio) as the mobile phase silica gel as the stationary phase, the product is collected and spin-dried, and dried in a vacuum at 80°C 24h, the product was obtained.
Embodiment 2
[0039]This example provides the synthesis of 4,4'-(10H-phenothiazine-2,7-diyl)dianiline:
[0040]
[0041] S1. Add 3.57g (0.01mol) 2,7-dibromo-10H-phenothiazine, 4.335g (0.025mol) p-aminophenyl borate hydrochloride into a 500ml three-necked flask, tetrahydrofuran (THF) as the solvent, and then add 2mol 37.5ml of potassium carbonate solution per L and an appropriate amount of Aliquat336, magnetic stirring and argon gas, after heating the oil bath to 75°C, add 0.020g tetrakistriphenylphosphine palladium, reflux reaction for 24h, add water to quench the reaction, and then use organic Solvent extraction, and then distill off the solvent under reduced pressure.
[0042] S2. The product after the step S1 is evaporated to remove the solvent is purified by column chromatography with dichloromethane:n-hexane=1:2 (volume ratio) as the mobile phase silica gel as the stationary phase, and the product is collected and spin-dried, and dried in a vacuum at 80°C 24h, the product was obtai...
Embodiment 3
[0044] This example provides the synthesis of 5,5'-(10H-phenothiazine-2,8-diyl)bis(thiophen-2-amine):
[0045]
[0046] S1. Add 3.57g (0.01mol) 2,8-dibromo-10H-phenothiazine, 3.574g (0.025mol) (5-aminothiophen-2-yl) boronic acid into a 500ml three-necked flask, tetrahydrofuran (THF) as solvent, Then add 2mol / L potassium carbonate solution 37.5ml and an appropriate amount of Aliquat336, stir magnetically and pass argon, heat the oil bath to 75°C, add 0.020g tetrakistriphenylphosphine palladium, reflux reaction for 24h, add water to quench the reaction, Then it was extracted with an organic solvent, and the solvent was distilled off under reduced pressure.
[0047] S2. The product after the step S1 is evaporated to remove the solvent is purified by column chromatography with dichloromethane:n-hexane=2:1 (volume ratio) as the mobile phase silica gel as the stationary phase, and the product is collected and spin-dried, and dried in a vacuum at 80°C 24h, get the product
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com