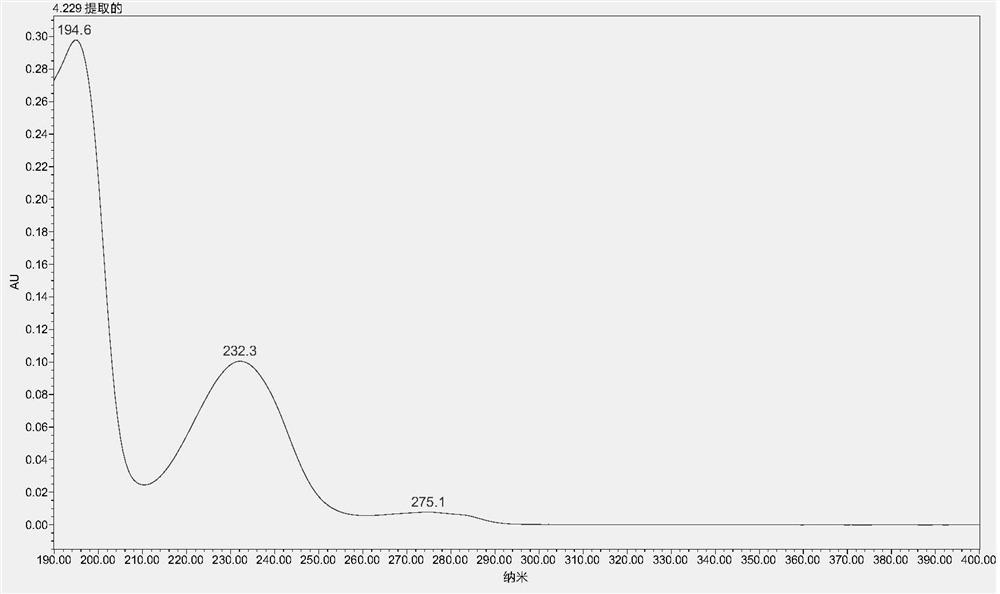
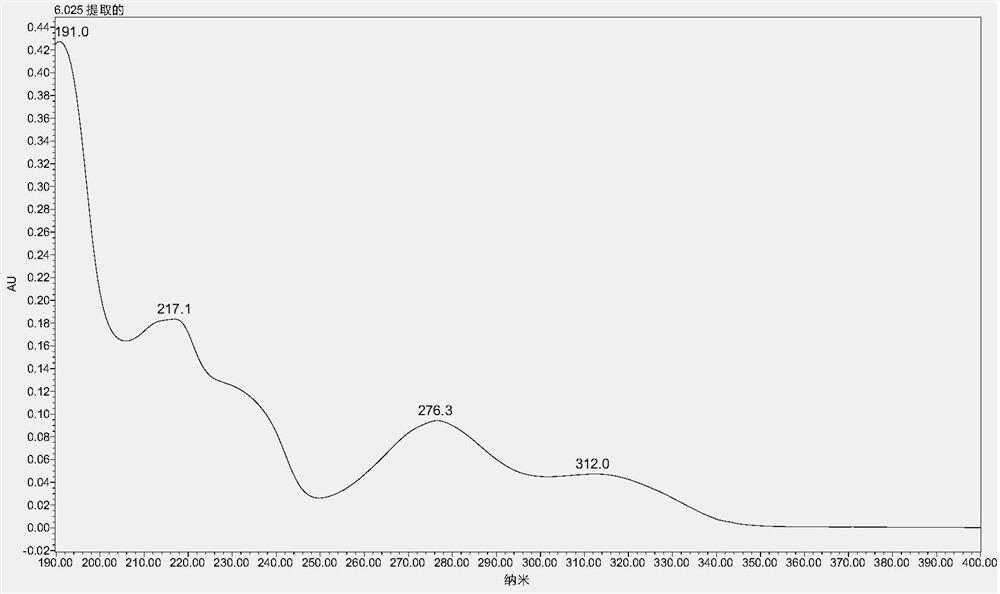
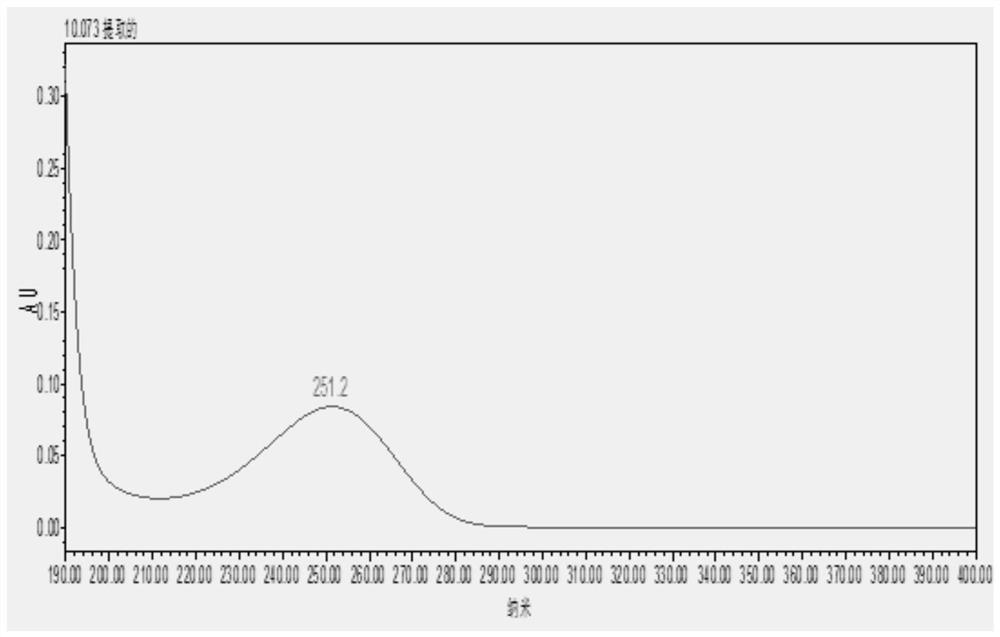
A method for detecting the contents of paeoniflorin, liquiritin and ammonium glycyrrhizinate in Chashao oral liquid
A technology of Chai Shao oral liquid and ammonium glycyrrhizinate, which is applied in the field of quality inspection of Chinese veterinary medicine preparations, can solve problems such as long inspection time, unstable quality, and unstable therapeutic effect of traditional Chinese medicine compound
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Embodiment 1 A kind of detection method of content of paeoniflorin, liquiritin and ammonium glycyrrhizinate in Chai Shao oral liquid
[0037] 1 Materials and methods
[0038] 1.1 Drug and reagent reference substances are China National Institutes for Food and Drug Control: Paeoniflorin reference substance: batch number: 110736-201438, content 96.4%; ammonium glycyrrhizinate reference substance, batch number 110731-201720, content 97.7%; liquiritin reference substance, batch number 11610-201106, content 93.7%. Acetonitrile and phosphoric acid are chromatographically pure; ethanol is analytically pure; ultrapure water. Chai Shao Oral Liquid is provided by Qingdao Weilan Biological Co., Ltd., a new veterinary drug research and development cooperation unit of the 2018 provincial key research and development plan project.
[0039] 1.2 Instrument Analytical balance: Sensitivity 0.00001g; Waters Acquity TM Ultra performance LC ultra-high performance liquid chromatography; ...
Embodiment 2
[0072] Example 2 Detection method of paeoniflorin, liquiritin and ammonium glycyrrhizinate in Chai Shao oral liquid Chai Shao oral liquid includes the following components by weight:
[0073] 12 parts of Bupleurum, 4 parts of Paeoniae Alba, 10 parts of Citrus aurantium, and 8 parts of Licorice.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com