Magnetic covalent organic framework material, and preparation method and application thereof in detection of perfluorinated compounds
A covalent organic framework and magnetic technology, applied in the field of environmental inspection, can solve the problems of low separation efficiency of PFCs, long preparation time, harsh reaction conditions, etc., and achieve the effects of rapid enrichment and separation, simple synthesis steps, and mild reaction conditions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0053] The third embodiment of the present invention provides an application of the above-mentioned magnetic covalent organic framework material for detecting perfluorinated compounds.
[0054] In one or more examples of this embodiment, the perfluoro compound is PFBS (perfluorobutane sulfonate), PFHpA (perfluoroheptanoic acid), PFOA, PFHxS (perfluorohexyl sulfonic acid), PFNA (Perfluorononanoic acid), PFDA (perfluorodecanoic acid), PFOS, PFUdA (perfluoroundecanoic acid), PFDoA (perfluorododecanoic acid).
[0055] The fourth embodiment of the present invention provides a method for enriching perfluorinated compounds in wastewater. The magnetic covalent organic framework material is added to wastewater containing perfluorinated compounds, and after shaking for a period of time, hysteresis separation is performed , The separated magnetic covalent organic framework material is eluted.
[0056] In one or more embodiments of this embodiment, the eluent is methanol, ethanol, acetone or ac...
Embodiment 1
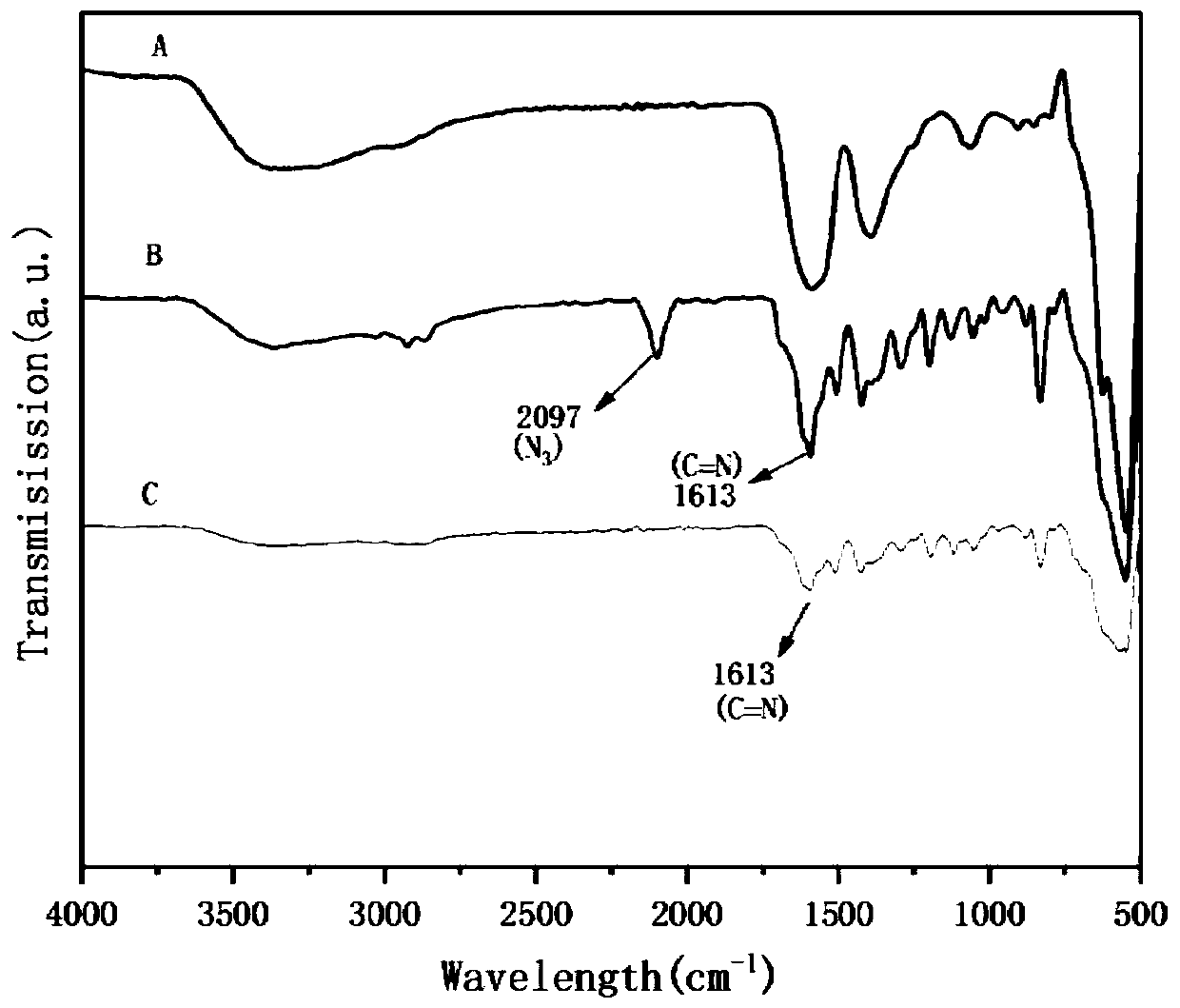
[0062] Synthesis of 2,5-bis(2-(2-azidoethoxy)ethoxy)-1,4-phthalaldehyde.
[0063] (1) Add 2-(2-chloroethoxy)ethanol (4g, 32mmol), deionized water (20mL) to a 50mL round-bottom flask, and then add sodium azide (5.2g, 80mmol, 2.5eq). Stir at 80°C for 18 hours under nitrogen protection, then pour into 35 mL sodium hydroxide solution (5% (W / V)) and extract with ethyl acetate (3×35 mL). The organic phase was dried with anhydrous sodium sulfate and spin-dried to obtain 2-(2-azidoethoxy)ethanol as an oil.
[0064] (2) Under the protection of nitrogen, add 2-(2-azidoethoxy)ethanol (2g, 15.3mmol) and dry dichloromethane (20mL) to a 100mL round bottom flask, and then add to the above solution Add triethylamine (2.7mL, 18.4mmol, 1.5eq), cool to 0°C, add p-toluenesulfonyl chloride (3.48g, 18.4mmol, 1.2eq). The reaction mixture was stirred at 0°C for 1 hour, warmed to room temperature and stirred for 17 hours until the reaction was complete. Then it was washed with sodium bicarbonate soluti...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com