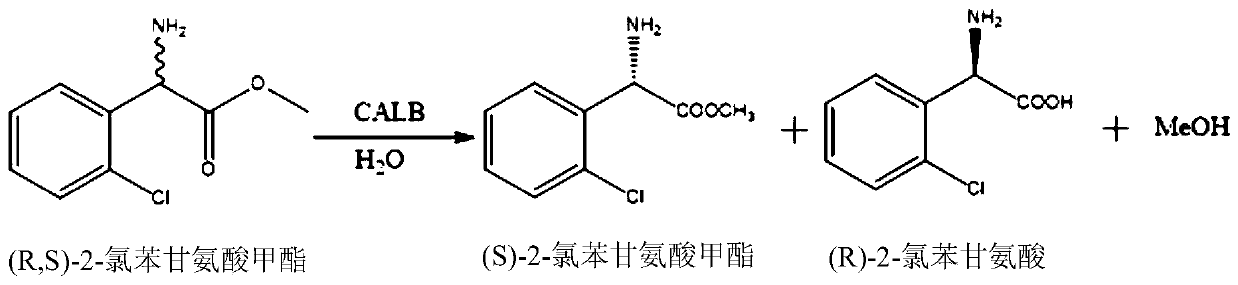
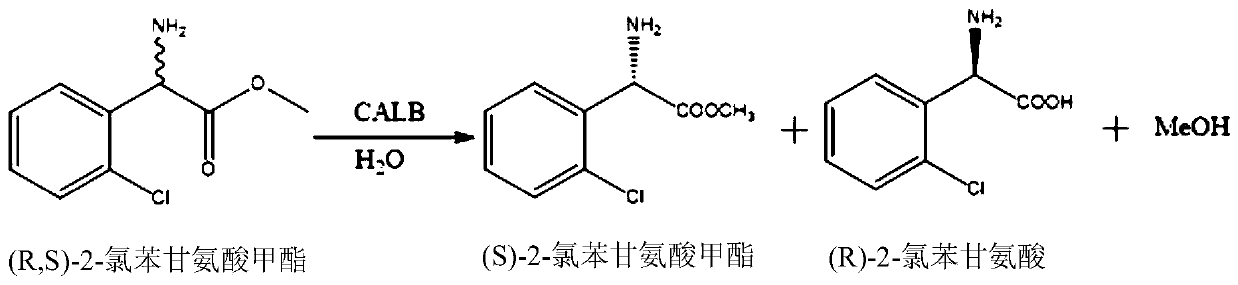
Lipase mutant and application thereof in preparation of (S)-2-chlorophenylglycine methyl ester
A technology of chlorophenylglycine methyl ester and mutants, applied in the fields of application, enzyme, hydrolase, etc., can solve the problems of long reaction time, achieve high selectivity, improve selectivity reversal, and shorten reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Example 1 Construction of wild-type Candida antarctica lipase B engineering bacteria (E.coli Rosetta(DE3) / pET22b(+)-CALB-WT)
[0045] 1. Cloning of wild-type Candida antarctica lipase B (CALB) gene
[0046] Pichia pastoris (Z. Liu. Cloning, expression and characterization of a lipase gene from the Candida antarctica ZJB09193 and its application in biosynthesis of vitamin A esters. Microbiol. Res., 2012, 167, 452 ~460) was inoculated in YPD liquid medium and cultured overnight, the fermentation liquid was centrifuged in a 2 mL centrifuge tube at 3000 rpm / min for 5 min, the supernatant was discarded, and the bacteria were collected; the genome was extracted using a fungal extraction kit.
[0047] According to the CALB gene (SEQ ID NO.1), primer 1: TTACCTAGTGGTTCCGACCC and primer 2: AGGAGTAACAATTCCTGAACAGG were designed, and the extracted genome was used as a template for PCR reaction to clone the wild-type CALB gene. The reaction system was as follows:
[0048]
[004...
Embodiment 2
[0068] Example 2 Induced expression of wild-type Candida antarctica lipase B (CALB-WT)
[0069] Inoculate the engineered bacteria E.coli Rosetta(DE3) / pET22b(+)-CALB-WT obtained in Part 3 of Example 1 into LB liquid medium containing 100 μg / mL ampicillin and 20 μg / mL chloramphenicol at 37° C. Cultivate overnight, inoculate in fresh LB medium containing a final concentration of 100 μg / mL ampicillin and 20 μg / mL with an inoculum volume concentration of 1% (v / v), culture at 37 °C and 180 rpm for 3 h, and then add to the culture medium Add IPTG at a final concentration of 0.1 mM, incubate at 22°C for 12 hours, and centrifuge at 10,000 g at 4°C for 10 minutes to obtain the corresponding bacterial cells.
[0070] The cells obtained above produce corresponding proteins, which can be used to prepare crude enzyme solution, and asymmetrically hydrolyze racemic 2-chlorophenylglycine methyl ester.
Embodiment 3
[0071] Example 3 Wild-type Candida antarctica lipase B (CALB-WT) asymmetrically hydrolyzes 2-chlorophenylglycine methyl ester
[0072] Add 0.5 g of racemic 2-chlorophenylglycine methyl ester and 50 mL of pH 7.0, 0.2 M sodium phosphate buffer in the reaction flask, then add 10 g of thalline cells obtained in Example 2 and mix evenly. The concentration of racemic 2-chlorophenylglycine methyl ester was 10 g / L, and the reaction was carried out at 30° C. with a controlled rotation speed of 600 rpm / min for 24 hours, and the reaction was terminated.
[0073] The reaction solution after the reaction was finished was analyzed by HPLC, and the results are shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com