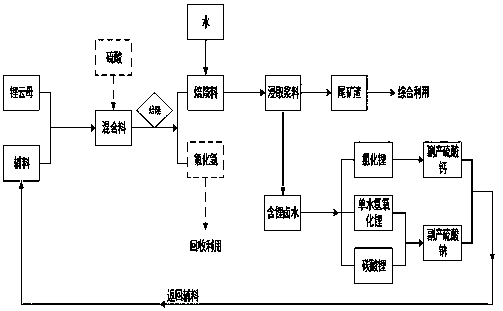
System method for extracting lithium-containing brine from lepidolite and manufacturing lithium salt
A lepidolite and brine technology, applied in lithium carbonate;/acid carbonate, silicate, lithium halide, etc., can solve the problems of long leaching time, poor selectivity, and product quality decline, etc. Achieve the effect of improving the comprehensive added value, the overall process is simple, and the product quality is stable
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074] (1) Take 300g of lepidolite with a lithium oxide content of 2.8%, add 90g of sodium sulfate and 60g of calcium sulfate, finely grind and mix evenly to obtain a mixture;
[0075] (2) Roast the mixture at 1050°C for 10 minutes to obtain the roasted material;
[0076] (3) After cooling, add 600mL of water, stir for 30min, and filter to obtain filtrate and filter residue;
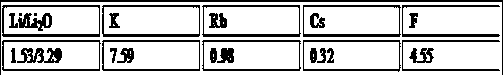
[0077] Table 1 Lepidolite data, %
[0078]
[0079] Table 2 filtrate data, g / L
[0080]
[0081] Table 3 Residue data, %
[0082]
[0083] (4) The obtained filtrate is lithium-containing brine, and the obtained filter residue is lithium extraction tailings.
Embodiment 2
[0085] (1) Take 300g of lepidolite with a lithium oxide content of 3.5%, add 60g of sodium sulfate, 60g of calcium sulfate, and 30g of concentrated sulfuric acid (98%), and mix evenly to obtain a mixture;
[0086] (2) Roast at 850°C for 30 minutes, absorb tail gas with a mixed solution of calcium carbonate and calcium hydroxide, and obtain roasted material and tail gas absorption slag;
[0087] (3) Add 600mL of water to the baking material, stir for 30min, and separate the solid and liquid to obtain the filtrate and filter residue;
[0088] (4) Solid-liquid separation of acid-absorbing tail gas slag;
[0089] Table 4 Lepidolite data, %
[0090]
[0091] Table 5 filtrate data, g / L
[0092]
[0093] Table 6 Residue data, %
[0094]
[0095] Table 7 Absorption acid tail gas slag data (approximate value), %
[0096]
[0097] (5) The obtained filtrate is lithium-containing brine, the obtained filter residue is lithium extraction tailings, and the obtained acid gas s...
Embodiment 3
[0099] (1) Take 10000g of lepidolite with a lithium oxide content of 3.9%, add 1500g of sodium sulfate and 1500g of calcium sulfate, finely grind and mix evenly, add 4000g of concentrated sulfuric acid (98%), and roast at 750°C for 300min to obtain a roasted material; The mixed solution of calcium carbonate and calcium hydroxide absorbs tail gas to obtain roasted material and tail gas slag;
[0100] (2) Add 20,000mL of water to the roasting material, stir for 30 minutes, and separate the solid and liquid to obtain the filtrate and filter residue;
[0101] (3) Solid-liquid separation of acid-absorbing tail gas slag;
[0102] Table 8 Lepidolite data, %
[0103]
[0104] Table 9 filtrate data, g / L
[0105]
[0106] Table 10 Residue data, %
[0107]
[0108] Table 11 Absorption acid tail gas slag data (approximate value), %
[0109]
[0110] (4) The obtained filtrate is the lithium-containing brine, the obtained filter residue is the lithium extraction tailings res...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com