Preparation method and application of cobalt organic macro-cyclic compound
A cyclic compound, metal-organic technology, applied in the field of supramolecular chemistry, can solve the problem of high reaction temperature, and achieve the effects of stable chemical properties, high yield and green dehydrogenation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
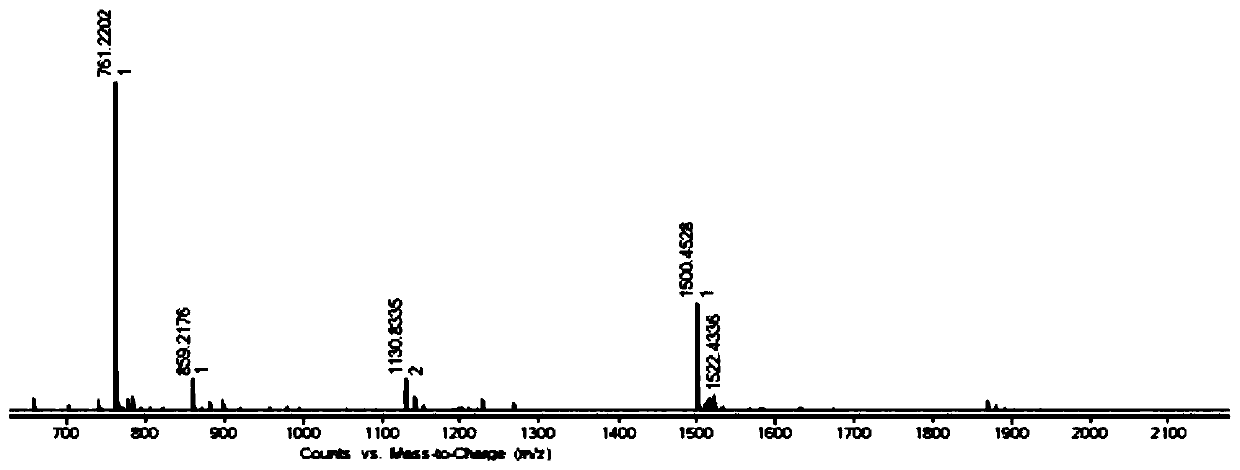
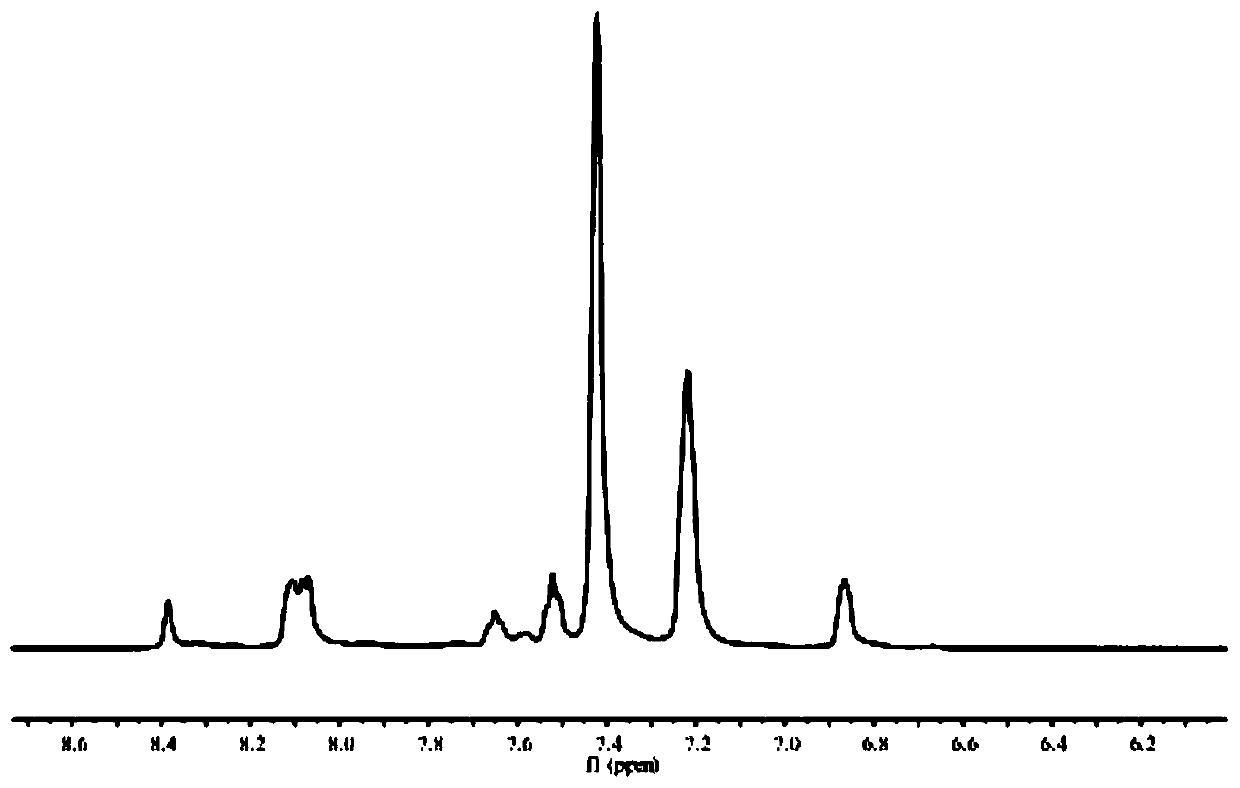
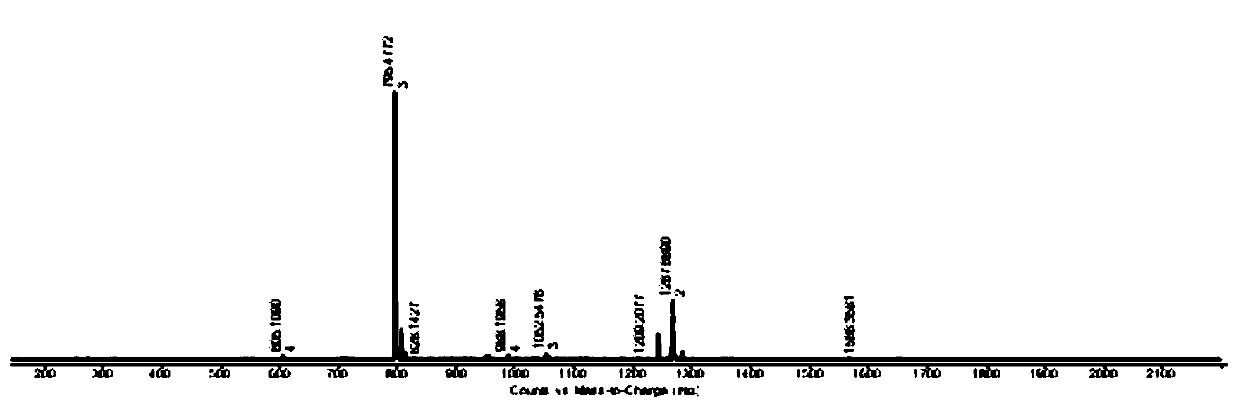
Image
Examples
Embodiment 1
[0028] Methyl isophthalate (1.94g, 0.01mol) and hydrazine hydrate (2g, 0.04mol) were added to 35mL of methanol solution, and reacted at 75°C for 25h. After the reaction was completed, the precipitate was collected by filtration while hot and dried. Obtain isophthalic acid dicarboxyl hydrazide compound; Will isophthalic acid dicarboxyl hydrazide compound (0.388g, 2mmol) and 2-diphenylphosphine benzaldehyde (1.16g, 4mmol) join the methanol solution that is added to 35mL 12 drops of glacial acetic acid were added dropwise and reacted at 75°C for 25h. After the reaction was completed, the precipitate was collected by filtration while hot and dried to obtain the ligand DTP (1.1g, yield 74%); the ligand DTP (73.8mg, 0.1mmol) and cobalt chloride hexahydrate (35.7mg, 0.15mmol) were added to 35mL methanol solution, reacted at 70°C for 25h, after the reaction was completed, 25mg ammonium hexafluorophosphate was added and stirred for 1.5h, then 70mL diethyl ether was added, After stirrin...
Embodiment 2
[0030] The ligand DTP (73.8mg, 0.1mmol) prepared in Example 1 and cobalt nitrate hexahydrate (29.1mg, 0.1mmol) were added to 30mL methanol solution, reacted at 70°C for 24h, after the reaction was completed, 20mg Ammonium hexafluorophosphate was stirred for 1 h, then 65 mL of ether was added, and after stirring and filtering, the target compound Co-DTP (26.4 mg, yield 30%) was obtained.
Embodiment 3
[0032] The ligand DTP (73.8mg, 0.1mmol) prepared in Example 1 and cobalt sulfate heptahydrate (70.3mg, 0.25mmol) were added to 40mL methanol solution and reacted at 65°C for 25h. After the reaction, 25mg heptahydrate was added Ammonium fluorophosphate was stirred for 1.5 h, then 70 mL of ether was added, and after stirring and filtering, the target compound Co-DTP (32.6 mg, yield 37%) was obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com