Novel iron-nickel-nitrogen co-doped carbon catalyst as well as preparation method and application thereof
A carbon catalyst and co-doping technology, applied in chemical instruments and methods, physical/chemical process catalysts, chemical/physical processes, etc., can solve the problem of slow charging process, unsatisfactory OER performance, and loss of competitiveness of zinc-air batteries. problems, to achieve uniform distribution of active sites, high stability, and well-developed pores.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0052] A preparation method of a novel iron-nickel-nitrogen co-doped carbon catalyst, the method comprising the steps of:
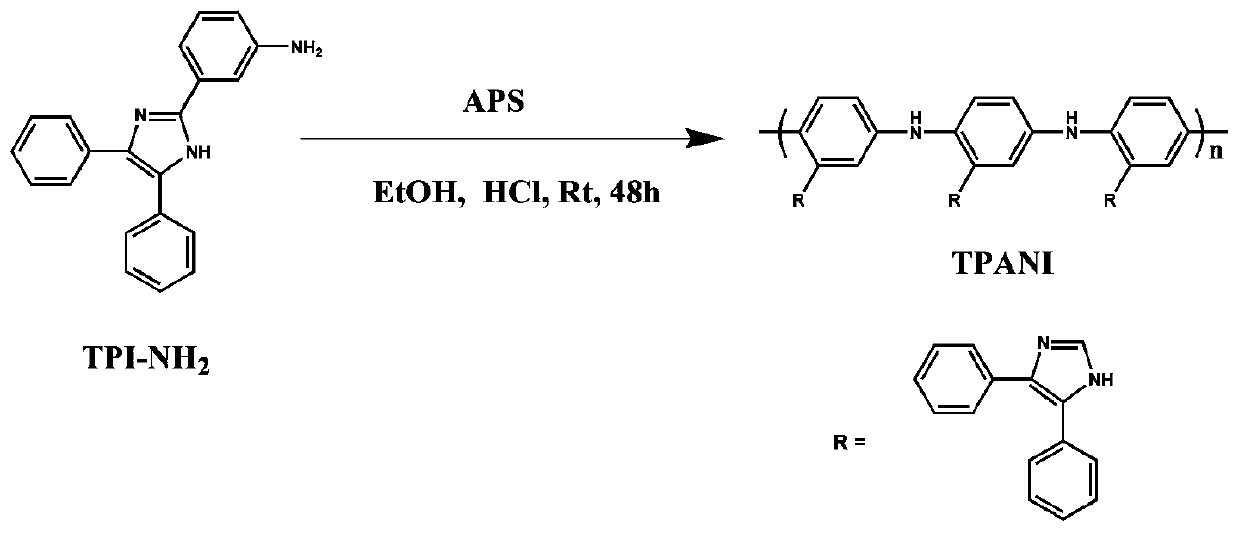
[0053] 1) The triaryl imidazolium aniline derivative (TPI-NH 2 ) is dissolved in a mixed solvent, and oxidative polymerization is carried out in the presence of an oxidizing agent to obtain a polyaniline derived polymer (TPANI).
[0054] 2) Dissolving the polyaniline-derived polymer (TPANI) obtained in step 1), iron source and nickel source in a solvent, and then adding melamine for mixing reaction to obtain a TPANI / melamine / Ni-Fe mixture.
[0055] 3) The TPANI / melamine / Ni-Fe mixture obtained in step 2) is heat-treated under the protection of a protective gas atmosphere to obtain an iron-nickel-nitrogen co-doped carbon catalyst (NiFe / N-C).
[0056] As preferably, step 1) is specifically: proportioning the triaryl imidazolium aniline derivative (TPI-NH 2 ) is dissolved in the first solvent. Then add an oxidizing agent (such as ammonium persulfate) and c...
Embodiment 1
[0066] 1) Preparation of polyaniline derived polymer (TPANI):
[0067] Weigh 4.0g (12.8mmol) TPI-NH 2Dissolve in a mixed solvent of 40ml of ethanol and 80ml of 1M HCl; then slowly drop into 60ml of 1M HCl aqueous solution with 2.98g (12.8mmol) of ammonium persulfate dissolved in it under stirring, and react at room temperature for 48h. After the reaction, the system was poured into a large amount of water, the product was collected by suction filtration, extracted with acetone for 48 hours, washed three times with 1M ammonia water and distilled water successively, and dried to obtain a triaryl imidazole polyaniline derivative polymer (TPANI).
[0068] 2) Preparation of TPANI / melamine / Ni-Fe mixture:
[0069] Weigh 2g (6.43mmol) TPANI and dissolve in THF / H 2 O mixed solvent (V THF / V H2O =2 / 1); then add 0.84g (2.88mmol) nickel nitrate hexahydrate and 0.38g (0.96mmol) ferric nitrate nonahydrate, reflux reaction 5h; then add 20g melamine again in reaction system and continue r...
Embodiment 2
[0073] 1) Preparation of polyaniline derived polymer (TPANI):
[0074] Weigh 5.0g (16mmol) TPI-NH 2 Dissolve in a mixed solvent of 50ml ethanol and 100ml 1M HCl; then slowly drop into 80ml of 1M HCl aqueous solution dissolved with 3.725g (16mmol) ammonium persulfate under stirring, and react at room temperature for 48h. After the reaction, the system was poured into a large amount of water, the product was collected by suction filtration, extracted with acetone for 48 hours, washed three times with 1M ammonia water and distilled water, and dried to obtain a triaryl imidazole polyaniline derivative polymer (TPANI).
[0075] 2) Preparation of TPANI / melamine / Ni-Fe mixture:
[0076] Weigh 2.4g (7.72mmol) TPANI and dissolve in THF / H 2 O mixed solvent (V THF / V H2O =2 / 1); then add 1.01g (3.46mmol) nickel nitrate hexahydrate and 0.46g (1.15mmol) ferric nitrate nonahydrate, reflux reaction 8h; then add 24g melamine to the reaction system and continue reflux reaction 8h. After the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com