Preparation method of fluorescent probe test paper for detecting lead content of soil
A fluorescent probe and lead content technology, which is applied in soil testing, measuring devices, fluorescence/phosphorescence, etc., can solve the problems of insufficient detection precision and high detection limit of the test paper method, and achieve easy operation, easy portability, and anti-interference good sex effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Synthesis of Embodiment 1 Organic Fluorescent Small Molecules
[0024] (1) Dissolve 8-hydroxyquinoline and hexamethylenetetramine in a methanol solvent in a molar ratio of 1:2, and react at reflux at 70°C for 2 hours. After cooling, rotary evaporation under reduced pressure is performed, and purified by chromatography to obtain 5,7-Dialdehyde-8-hydroxyquinoline.
[0025] (2) Dissolve 3-iodopropane-1-ammonia and 2-methylbenzothiazole in n-butanol at a molar ratio of 3:1, and react in a sealed high-pressure bottle at 60°C for 2-6 hours to obtain a yellow color The solid 3-(3-aminopropyl)-2-methylbenzothiazole was filtered, washed twice with 1 equivalent of diethyl ether, and then dried. The yield was above 98%.
[0026] (3) React 5,7-dialdehyde-8-hydroxyquinoline and twice the amount of 3-(3-aminopropyl)-2-methylbenzothiazole in n-butanol system, add organic base Triethylamine is the condensation catalyst. React for 2-6 hours at 120°C. When the system starts to condense...
Embodiment 2
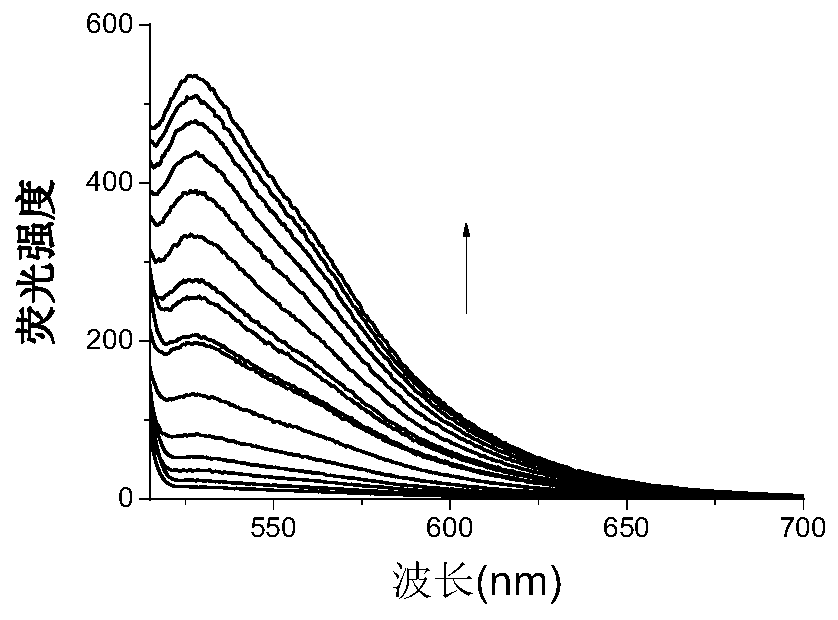
[0027] Example 2 is a graph showing the titration of heavy metal lead ions by small organic molecule fluorescent compounds in Tris-HCl.
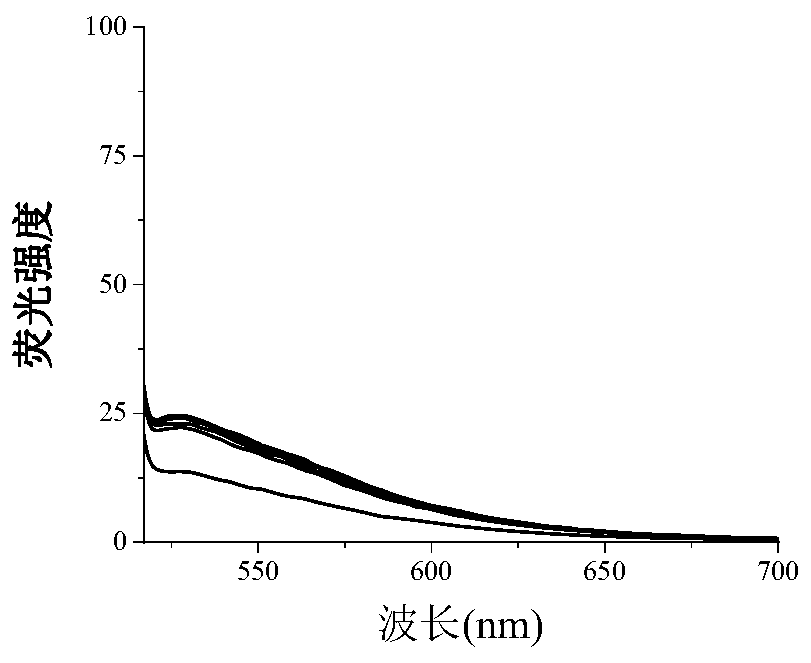
[0028] The 5mM organic small molecule fluorescent compound stock solution of the present invention is diluted to a concentration of 5 μM, and then heavy metal lead ions of different concentrations are added and measured by a fluorescence spectrophotometer (slit width=10, scanning speed=200nm, Ex=526nm) Its respective fluorescence intensity, the titration solution is Tris-HCl solution, finds that this organic small molecule fluorescent compound and heavy metal lead ion have very strong fluorescent effect. After the organic small molecule fluorescent compound is combined with the heavy metal lead ion, the fluorescence intensity increases significantly with the increase of the metal concentration, and when the concentration reaches saturation, the concentration will no longer increase, such as figure 2 shown. However, when organic small molec...
Embodiment 3
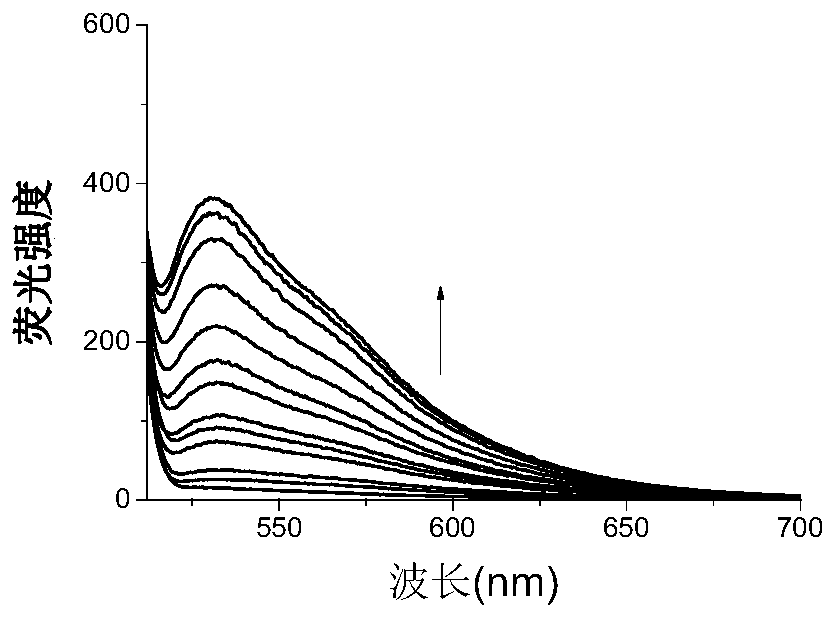
[0029] Example 3 is a graph showing the titration of heavy metal lead ions by small organic molecule fluorescent compounds in acetone.
[0030] The 5mM organic small molecule fluorescent compound stock solution of the present invention is diluted to a concentration of 5 μM, and then heavy metal lead ions of different concentrations are added and measured by a fluorescence spectrophotometer (slit width=10, scanning speed=200nm, Ex=526nm) Their respective fluorescence intensity, the titration solution is an acetone solution, and it is found that the organic small molecule fluorescent compound and the heavy metal lead ion have a strong fluorescent effect. After the organic small molecule fluorescent compound is combined with the heavy metal lead ion, the fluorescence intensity increases significantly with the increase of the metal concentration. When the local concentration reaches saturation, the concentration no longer increases, but the fluorescence intensity is about 40% lower...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com