In-vitro liver function detection method and system
A liver function and detection method technology, which is applied in the field of medical diagnosis, can solve the problems that static values cannot be used to reflect liver function, large individual differences in indicators, and large deviations in physiological indicators, etc., and achieve the effect of overcoming the inability to quantitatively detect liver function
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] In the experiment with 35-45kg Bama pigs, blood-donating pigs (n=6) were anesthetized and placed in the abdominal aorta and inferior vena cava, and about 1500ml of blood was released. The blood was filtered for white blood cells, and then stored at 4°C. Donor liver pigs (n=6) were anesthetized and placed catheters in the abdominal aorta, inferior vena cava, and portal vein, and instilled UW solution at 4°C through the abdominal aorta and portal vein. The dissociated liver was taken out and stored in UW solution at 4°C. Then the arteries, veins, and bile ducts of the liver are connected to the relevant pipelines of the isolated liver mechanical perfusion apparatus, and the pipelines are pre-filled with perfusate. The composition of the perfusion fluid is: 1.5L of whole blood, 150ml of 4% serum albumin, 18ml of 3% sodium bicarbonate, 9ml of 8% calcium chloride, 4000U heparin, 1g of cefoxitin, 500mg of metronidazole, and 15mg of bile salts; In addition, according to the p...
Embodiment 2
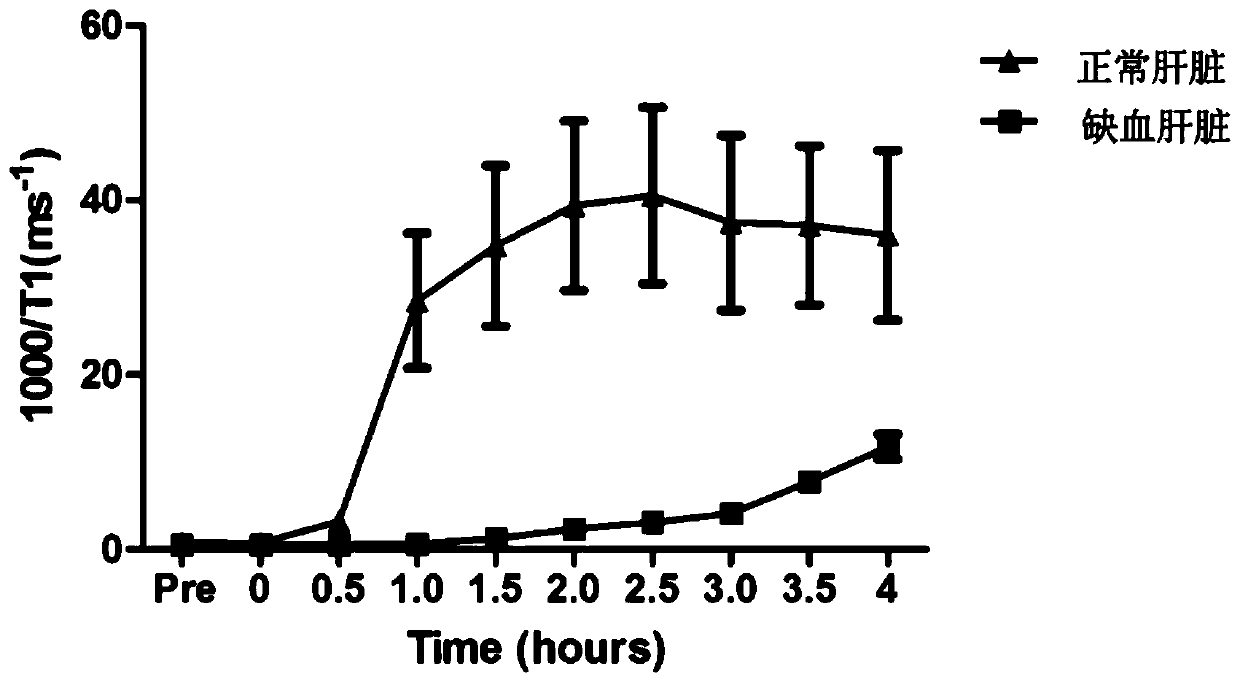
[0058] Normal group: 35-45kg Bama pigs were used for the experiment. The blood-donating pigs (n=6) were anesthetized and placed in the abdominal aorta and inferior vena cava, and about 1500ml of blood was released. The blood was filtered for white blood cells, and then stored at 4°C. Donor liver pigs (n=6) were anesthetized and placed catheters in the abdominal aorta, inferior vena cava, and portal vein, and instilled UW solution at 4°C through the abdominal aorta and portal vein. The dissociated liver was taken out and stored in UW solution at 4°C. Then the arteries, veins, and bile ducts of the liver are connected to the relevant pipelines of the mechanical perfusion apparatus for the isolated liver, and the pipelines are pre-filled with the above-mentioned perfusate. The perfusate consists of: 1.5L of whole blood, 100ml of 6% serum albumin, 24ml of 2% sodium bicarbonate, 5ml of 12% calcium chloride, 6000U of heparin, 1g of cefoxitin, 500mg of metronidazole, and 25mg of bile...
Embodiment 3
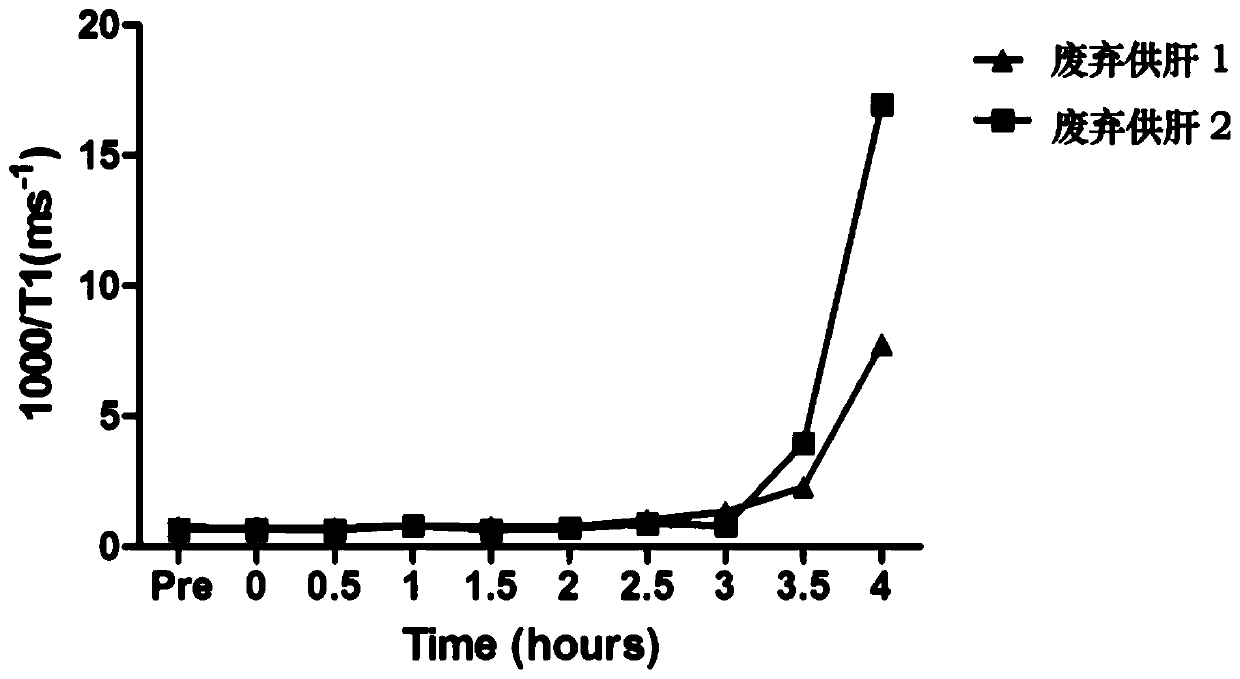
[0064] Abandoned human liver donors (n=2) were stored in cold storage for about 6 hours, taken out from the 4°C cold storage box, and the arteries, veins, and bile ducts of the liver were connected to the relevant pipelines of the isolated liver mechanical perfusion apparatus. Fill with the above perfusate. The composition of the perfusion solution is: 1.5L of whole blood, 130ml of 5% serum albumin, 21ml of 2.5% sodium bicarbonate, 7ml of 10% calcium chloride, 5000U heparin, 1g of cefoxitin, 500mg of metronidazole, and 20mg of bile salts; In addition, according to the pH of the perfusate, regular insulin and 2.5% sodium bicarbonate were used as appropriate, and the pH of the perfusate was adjusted within the range of 7.3 to 7.5.
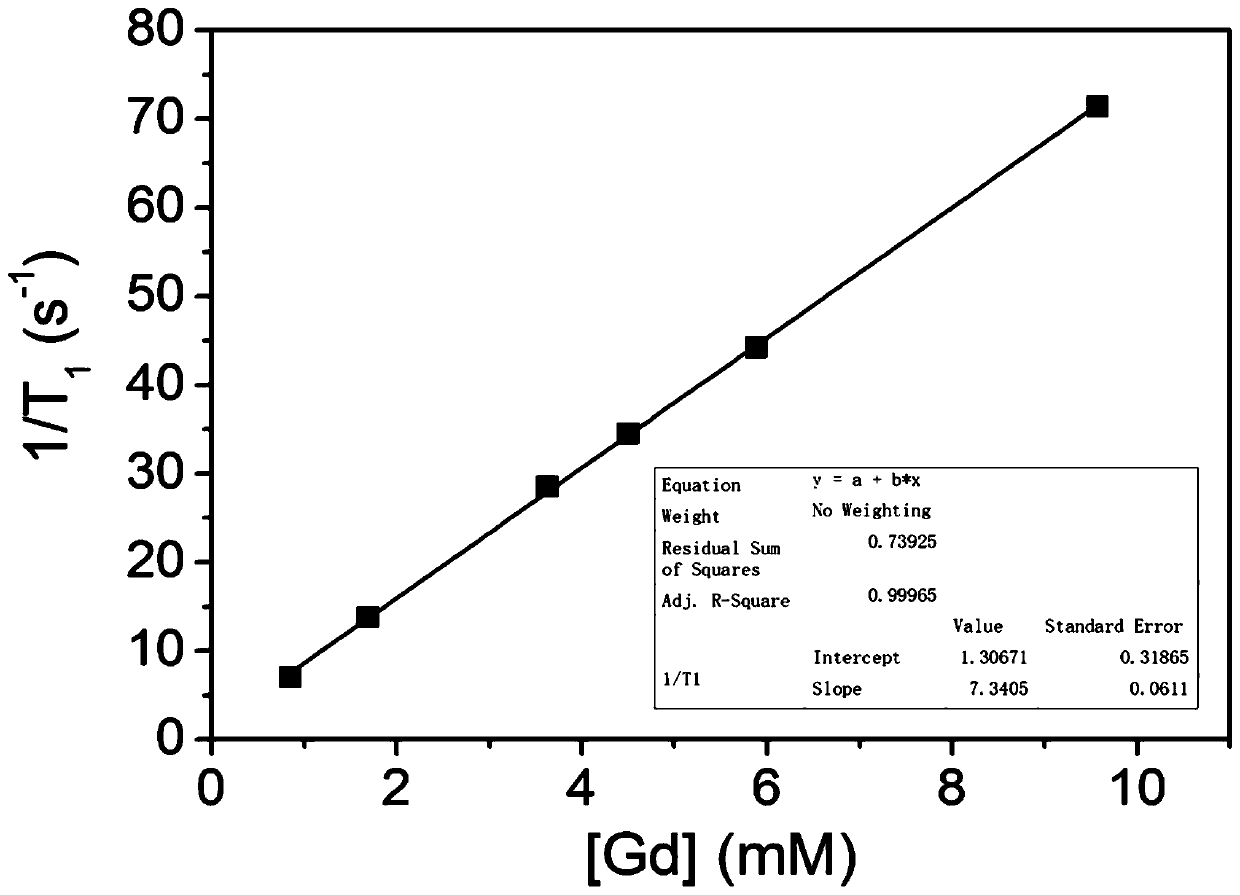
[0065] After 2 hours of perfusion, when the bile outflow is stable, inject gadoxetic acid disodium into the portal circulation, 0 hour, 0.5 hour, 1 hour, 1.5 hour, 2 hours, 2.5 hours, 3 hours, 3.5 hours, 4 hours, After 4.5 hours and 5 hours, take ou...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Magnetic field strength | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com