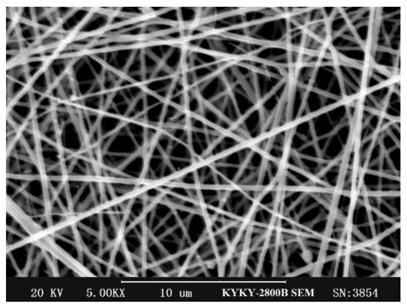
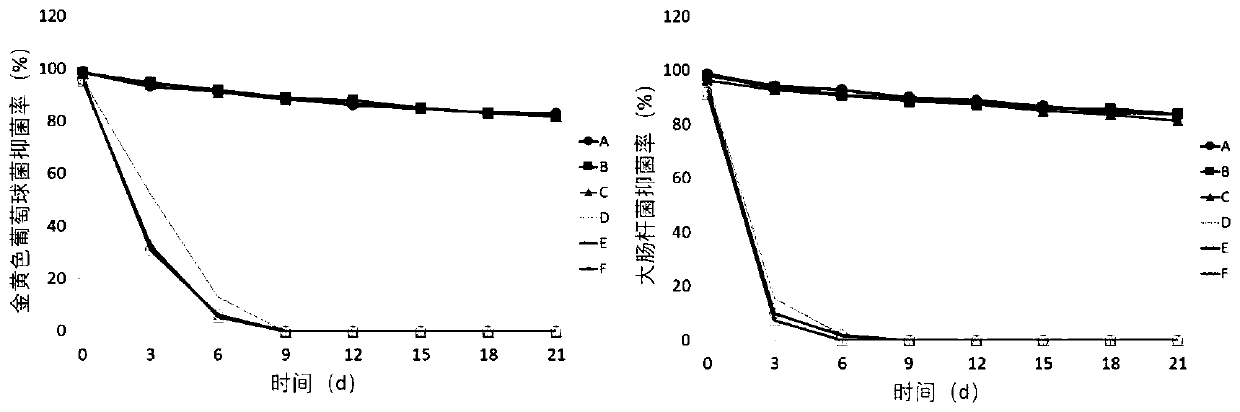
Chitosan-modified nanofiber slow-release antibacterial film and preparation method thereof
A chitosan modification and nanofiber technology, which is applied in fiber treatment, fiber chemical characteristics, rayon manufacturing, etc., can solve the problem of poor binding fastness between antibacterial agents and fibers, limiting the durability of antibacterial effects, swelling and disintegration, etc. problem, to achieve the effect of lasting effect, increasing content and increasing lipophilicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] A preparation method of chitosan modified nanofiber sustained-release antibacterial film, comprising the steps of:
[0027] 1. Add 0.7g of soybean lecithin, 0.2g of cholesterol and 0.4ml of terpinen-4-ol into 20ml of chloroform, stir well to dissolve it completely, remove the chloroform by rotary evaporation at room temperature, and then use a syringe at a rate of 5ml / min Inject it into 6 times the volume of water containing 3g / L Tween 80, then homogenize at a speed of 11000r / min, dialyze and concentrate to 1 / 6 volume with 15g / L polyvinyl alcohol 6000 solution to obtain pine oil En-4-ol liposomes;
[0028] 2. Add 1g of chitosan powder with a relative molecular weight of 30kDa and a deacetylation degree of 90%, 1.5g of benzenesulfonyl chloride, 1.5g of sodium hydroxide, 0.2g of 15-crown ether-5 and 0.15g of DMAP into 60ml of dichloromethane React at 50°C for 8 hours, then wash the reactant with a sodium hydroxide solution with a concentration of 8g / L, and collect the pr...
Embodiment 2
[0031] A preparation method of chitosan modified nanofiber sustained-release antibacterial film, comprising the steps of:
[0032] 1. Add 0.5g of soybean lecithin, 0.1g of cholesterol and 0.2ml of terpinen-4-ol into 20ml of chloroform, stir well to dissolve it completely, remove the chloroform by rotary evaporation at room temperature, and then use a syringe at a rate of 3ml / min Inject it into 5 times the volume of water containing 2g / L Tween 20, then homogenize at a speed of 10000r / min, dialyze and concentrate to 1 / 5 volume with 10g / L polyvinyl alcohol 6000 solution to obtain pine oil En-4-ol liposomes;
[0033] 2, 1g relative molecular weight is 20kDa, and deacetylation degree is 80% chitosan powder, 1g benzenesulfonyl chloride, 1g sodium hydroxide, 0.1g 15-crown ether-5 and 0.1g DMAP add 50ml dichloromethane at 40 React at ℃ for 6 hours, then wash the reactant with a sodium hydroxide solution with a concentration of 5g / L, and collect the precipitate by centrifugation;
[...
Embodiment 3
[0036] A preparation method of chitosan modified nanofiber sustained-release antibacterial film, comprising the steps of:
[0037] 1. Add 0.8g of soybean lecithin, 0.3g of cholesterol and 0.5ml of terpinen-4-ol into 20ml of chloroform, stir well to dissolve it completely, remove the chloroform by rotary evaporation at room temperature, and then use a syringe at a rate of 7ml / min. Inject it into 8 times the volume of water containing 5g / L Span 80, then homogenize at a speed of 12000r / min, dialyze and concentrate to 1 / 8 volume with 20g / L polyvinyl alcohol 6000 solution to obtain pine oil En-4-ol liposomes;
[0038] 2, 1g relative molecular weight is 50kDa, and deacetylation degree is 100% chitosan powder, 2g benzenesulfonyl chloride, 1.8g sodium hydroxide, 0.3g 18-crown ether-6 and 0.2g DMAP add 75ml dichloromethane in React at 60°C for 10 hours, then wash the reactant with a sodium hydroxide solution with a concentration of 10g / L, and collect the precipitate by centrifugation;...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com