Nitrogen-phosphorus-doped carbon composite iron phosphide three-dimensional rod-shaped porous material, lithium battery separator and preparation method, lithium-sulfur battery and electrical equipment
A lithium battery separator and porous material technology, which is applied in the field of lithium-sulfur batteries and electrical equipment, nitrogen-phosphorus-doped carbon composite iron phosphide three-dimensional rod-shaped porous materials, can solve problems such as not being able to be solved well, and achieve stable product performance , good diffusion, low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
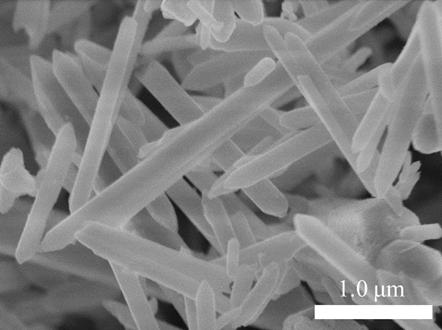
[0057] Weigh iron powder and 1,2,3-trimesic acid and put them into a hydrothermal reaction kettle lined with polytetrafluoroethylene, add hydrofluoric acid, nitric acid and pure water, and the molar ratio of each sample is n( iron powder): n(1,2,3-trimesic acid): n(HNO 3 ):n(HF):n(pure water)=3:2:2:7:800, after stirring evenly, place the hydrothermal reactor at 160 ℃ and seal it for 12 hours, cool it down to room temperature naturally, and use deionized Water, DMF, and absolute ethanol were washed three times each, and dried in an oven to obtain iron source A for use.
[0058] figure 1 Electron microscope image of the iron source material prepared in Example 1 of the present invention.
[0059] Weigh 200 mg of iron source A material, dissolve it in 30 mL of ethanol solution, then weigh 834 mg of sodium phytate and 60 mg of urea, stir overnight, and dry in a vacuum oven at 60 °C to obtain the precursor material. A certain mass of precursor material was weighed, placed in a s...
Embodiment 2
[0064] Weigh iron powder and 1,2,3-trimesic acid and put them into a hydrothermal reaction kettle lined with polytetrafluoroethylene, add hydrofluoric acid, nitric acid and pure water, and the molar ratio of each sample is n( iron powder): n(1,2,3-trimesic acid): n(HNO 3 ):n(HF):n(pure water)=6:4:4:10:1000, after stirring evenly, place the hydrothermal reactor at 160 ℃ and seal it for 12 hours, cool it down to room temperature naturally, and use deionized Water, DMF, and absolute ethanol were washed three times each, and dried in an oven to obtain iron source A for use.
[0065] Weigh 200 mg of iron source A material, dissolve it in 30 mL of ethanol solution, then weigh 662 mg of potassium phytate and 90 mg of ethylenediamine, stir overnight, and dry in a vacuum oven at 60 ℃ to obtain the precursor material . A certain mass of precursor material was weighed, placed in a square porcelain boat, and then annealed at 800 °C for 3 hours at a heating rate of 3 °C / min in a nitrogen...
Embodiment 3
[0069] Weigh iron powder and 1,2,3-trimesic acid and put them into a hydrothermal reaction kettle lined with polytetrafluoroethylene, add hydrofluoric acid, nitric acid and pure water, and the molar ratio of each sample is n( iron powder): n(1,2,3-trimesic acid): n(HNO 3 ):n(HF):n(pure water)=4:3:3:5:600, after stirring evenly, place the hydrothermal reactor at 160 ℃ and seal it for 12 hours, cool it down to room temperature naturally, and use deionized Water, DMF, and absolute ethanol were washed three times each, and dried in an oven to obtain iron source A for use.
[0070] Weigh 200 mg of iron source A material, dissolve it in 30 mL of ethanol solution, then weigh 344 mg of zinc phytate and 120 mg of dimethylamine, stir overnight, and dry in a vacuum oven at 60°C to obtain the precursor material . A certain mass of precursor material was weighed, placed in a square porcelain boat, and then annealed at 900 °C for 5 hours at a heating rate of 5 °C / min in an argon atmospher...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com