Double-metal cyanide catalyst for copolymerization of carbon dioxide and epoxide and preparation method of double-metal cyanide catalyst
A double metal cyanide and epoxy technology, applied in the chemical field, can solve the problems of uneconomical recycling of chelating agents, high catalyst manufacturing costs, and impact on industrial applications, etc., to achieve shortened induction period, low production cost, and high yield Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
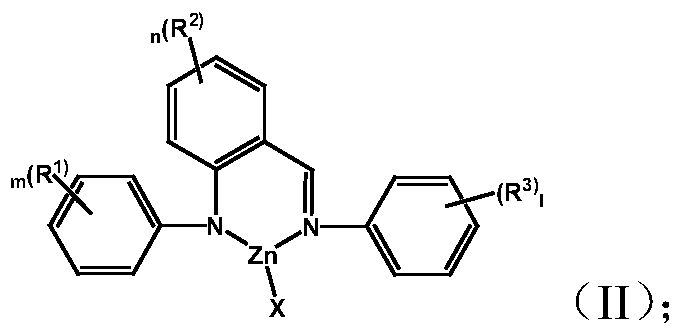
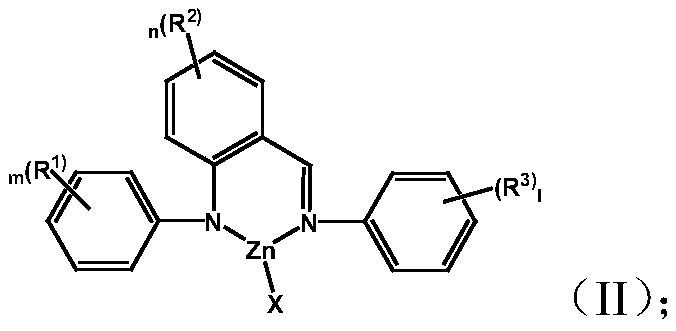
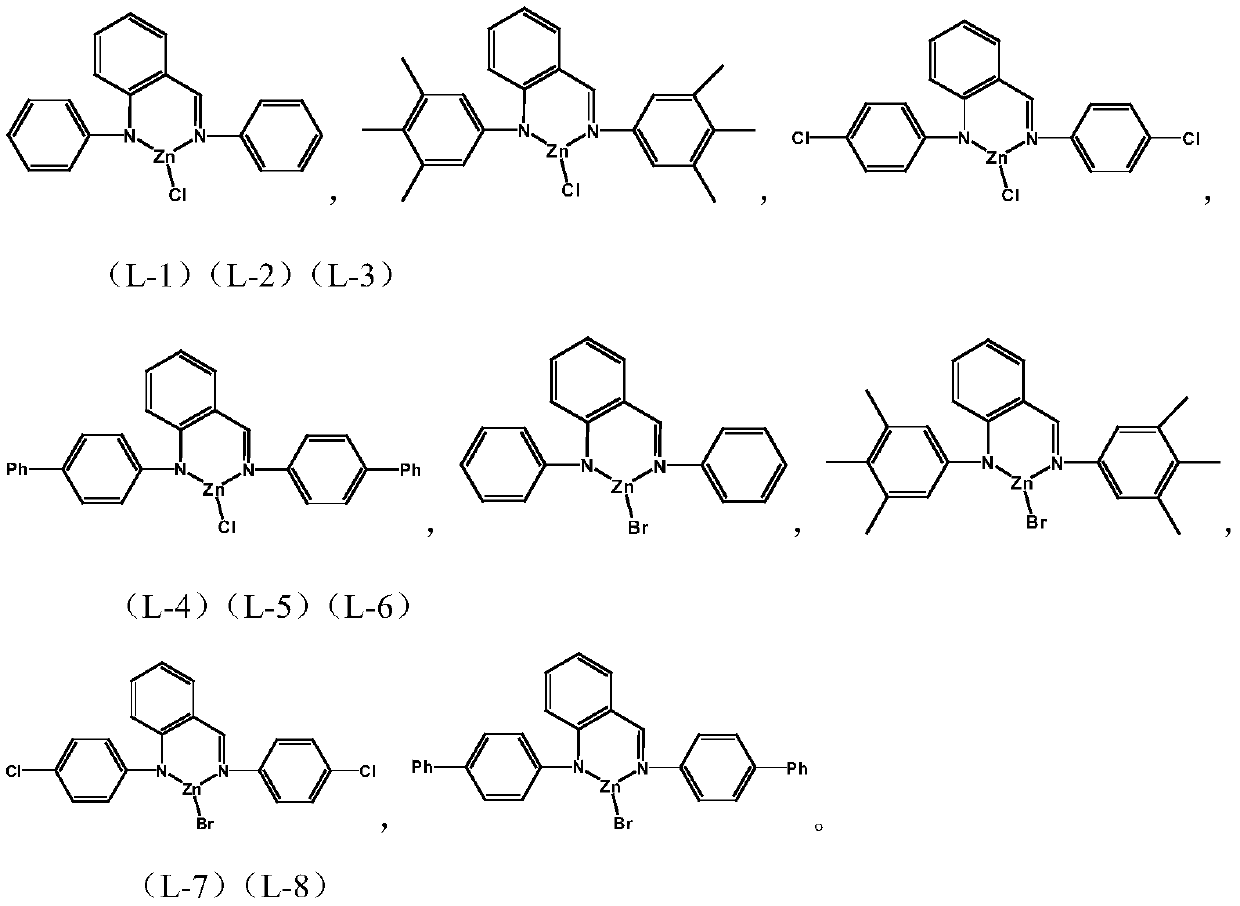
[0038] Complex M 1 Preparation of LX:
[0039] (1)a) Preparation of complex L-1:
[0040]
[0041] Under a nitrogen atmosphere, dissolve 0.3 g of (2-phenyliminomethylphenyl) aniline in 18 mL of toluene, add an equivalent amount of zinc chloride at -78 ° C, stir at room temperature overnight, and remove Solvent, the crude product was recrystallized from dichloromethane and cyclohexane. 0.42g of the product was obtained. Yield: 90.1%.
[0042] Elemental analysis structure: C: 61.22; H: 4.10; Cl: 9.55; N: 7.47; Zn: 17.43
[0043] b) Preparation of complex L-5:
[0044]
[0045] The complex L-5 was prepared by the same method as above, wherein the reactant zinc bromide was replaced with zinc chloride in a), and the yield was 93.7%.
[0046] Elemental analysis structure: C: 54.63; H: 3.81; Br: 19.11; N: 6.62; Zn: 15.71 (2) complexes L-2, L-3, L-4, L-6, L-7, L- Preparation of 8:
[0047] The complexes L-2, L-3, L-4, L-6, L-7, and L-8 were prepared by a similar method i...
Embodiment 2-9
[0051] Preparation of DMC catalyst:
[0052] Weigh a certain amount of metal M 1 Complex M 1 LX and metal salt M 2 e x f , Metal M 2 Add the cyanide in the ball mill tank respectively, add a small amount of solvent (ethylene glycol / glycerol / tert-butanol / acetone), and then put steel balls. The ball mill jar is placed in a ball mill with a rotational speed of 25-60 Hz to run for 10-60 minutes. After the catalyst is ground, it is washed several times with a mixed solution of alcohol solvent and water, filtered, and the white insoluble matter is dried to a constant weight in a vacuum oven at 60°C, and ground for later use. The preparation parameters of Examples 2-9 are shown in Table 2.
[0053] Table 2
[0054]
Embodiment 10~17
[0056] All reaction conditions, parameters and product parameters are as shown in Table 3, and the reaction steps are summarized as follows:
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com