Long-acting composite enzyme disinfection composition for children and application thereof
A composite enzyme and composition technology, applied in the fields of biotechnology and medical and health care, can solve the mechanism of action, side reactions have not been clinically verified, biological enzyme preparations cannot achieve sustained-release long-acting antibacterial, and yeast and fungal contamination cannot be achieved. Control and other problems to achieve the effect of no skin coloring problems, strong durability and prolonged activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1: Preparation of Complex Enzyme Inclusion Complex
[0024] In order to easily distinguish the groups of inclusion complexes, lysozyme in various compound enzyme dextrin inclusion complexes is abbreviated as A, lysozyme as B, chitinase as C, and helicase as D.
[0025] Preparation method of ABCD-type compound enzyme dextrin inclusion compound: Accurately weigh 2g of lysozyme (20000U / mg), 2mg of lysoglucoccus enzyme (1200U / mg), 2g of chitinase (100U / mg), and 0.2g of helicase , add 200ml of purified water to fully dissolve. Add it dropwise into 400ml of 4% hydroxypropyl β-cyclodextrin solution at a speed of 20ml / min, stir magnetically at 300rpm for 5min at room temperature, and ultrasonically for 10s, repeat the above steps 2 to 3 times, and transfer to vacuum rotary evaporation In the instrument, adjust the temperature to 40-60°C, and continue to rotate and stir for 4-10h. Transfer to a vacuum lyophilizer for lyophilization. The ABCD type compound enzyme dextr...
Embodiment 2
[0032] Example 2: Verification of the stability of complex enzyme inclusion complex
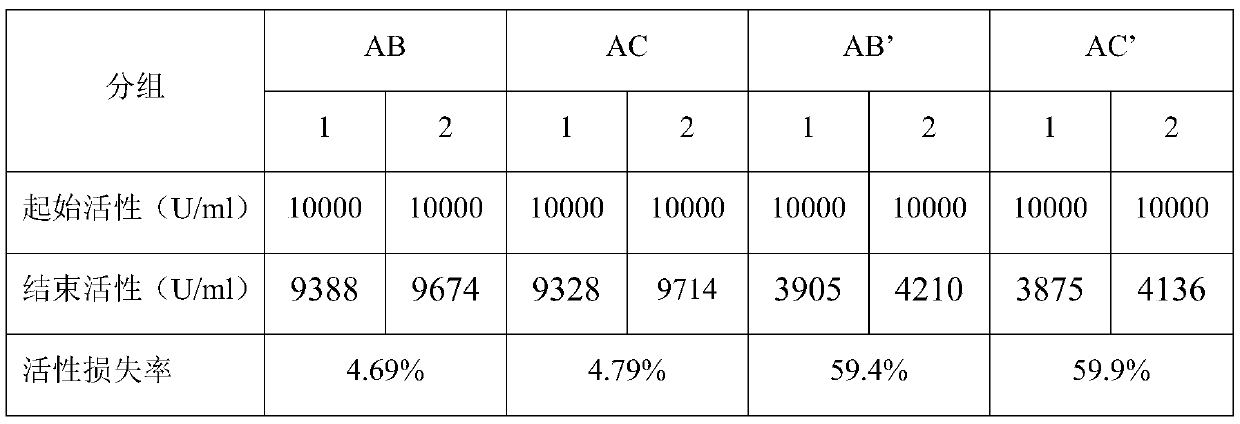
[0033] Select the AC-type compound enzyme in Example 1 and the AB-type compound enzyme in Comparative Example 1 as the experimental object, configure 0.05% solution, and simultaneously use the same activity of the unincluded AB and AC composition (marked as AB' and AC') The solution was used as a reference, and the activity of lysozyme in the above combination was used as an index to consider the stability of the compound enzyme in the inclusion compound. The above compound enzyme solutions were placed in an environment of 40°C for accelerated aging for 129 days, and the activities of the two solutions were measured respectively (equivalent to 2 years of storage at room temperature), and each group of experiments was repeated twice. The comparison is shown in Table 1.
[0034] Table 1 Stability test of inclusion compound
[0035]
[0036] It can be confirmed through tests that the comple...
Embodiment 3
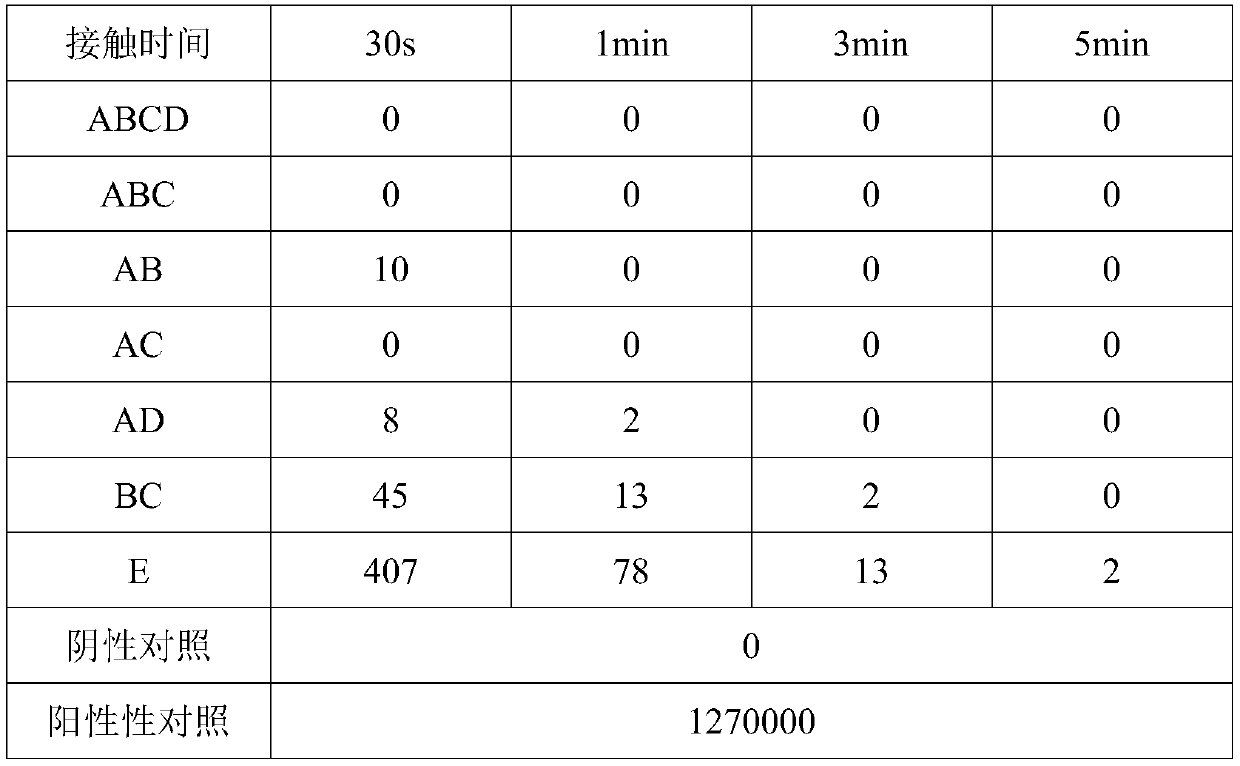
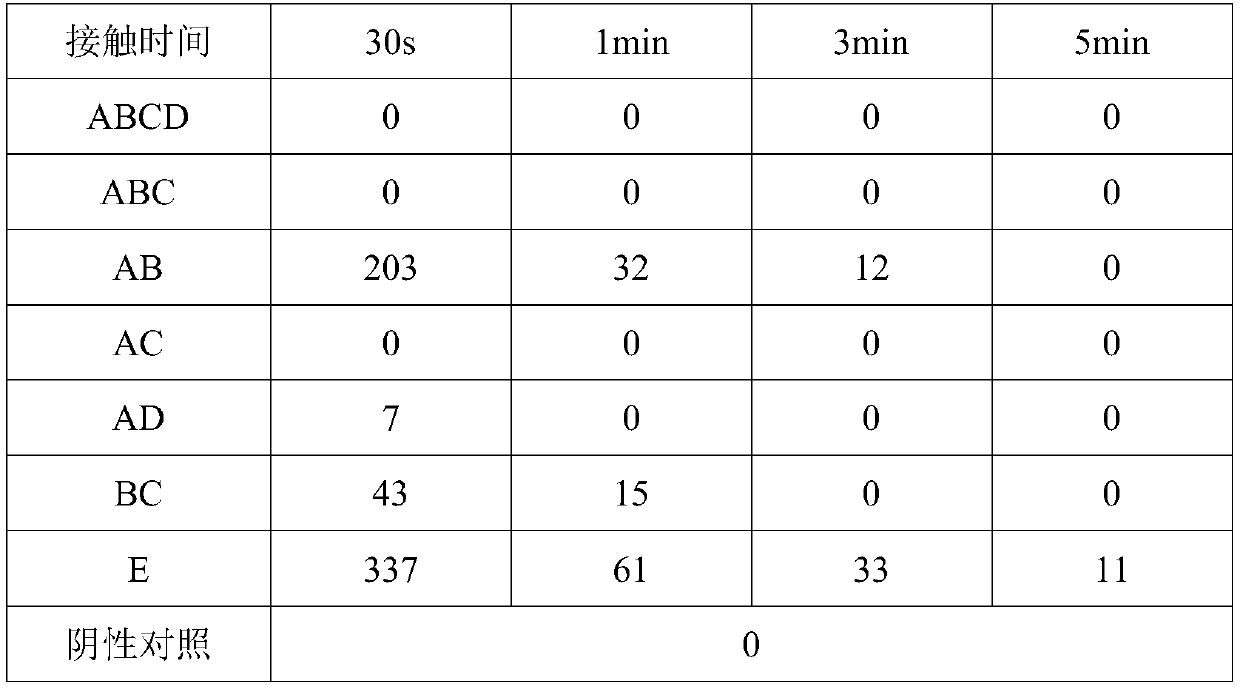
[0037] Embodiment 3: verification of disinfection effect
[0038] Take Staphylococcus aureus and Candida albicans respectively as the representatives of clinical pathogenic microorganisms to measure the disinfection effect verification of the present invention. Separately configure the bacterial suspensions of Staphylococcus aureus and Candida albicans so that the concentration of the bacterial suspensions is 1-10×10 6 cfu / mL, cultured after serial dilution was used as a positive control, and a blank plate was used as a negative control. Get respectively 1g various types of compound enzyme dextrin inclusion complex ABCD, ABC, AC, AD or BC in embodiment 1, or 1g AB type compound enzyme dextrin inclusion compound, polyhexamethylene biguanide (PHMB ) 1g (20% w / w), trehalose 1g, add 1000g of purified water, mix well and make a spray form, that is, become a disinfectant spray ABCD, ABC, AC, AD, BC, AB, at the same time only add PHMB and The solution of the stabilizer was used as ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com