Method for removing impurities from waste battery leachate
A technology for waste batteries and leaching solution, applied in the direction of improving process efficiency, can solve the problems of high loss rate of valence metals, low production efficiency, long adsorption time, etc., and achieve the effect of saving auxiliary material cost, fast filtration speed and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
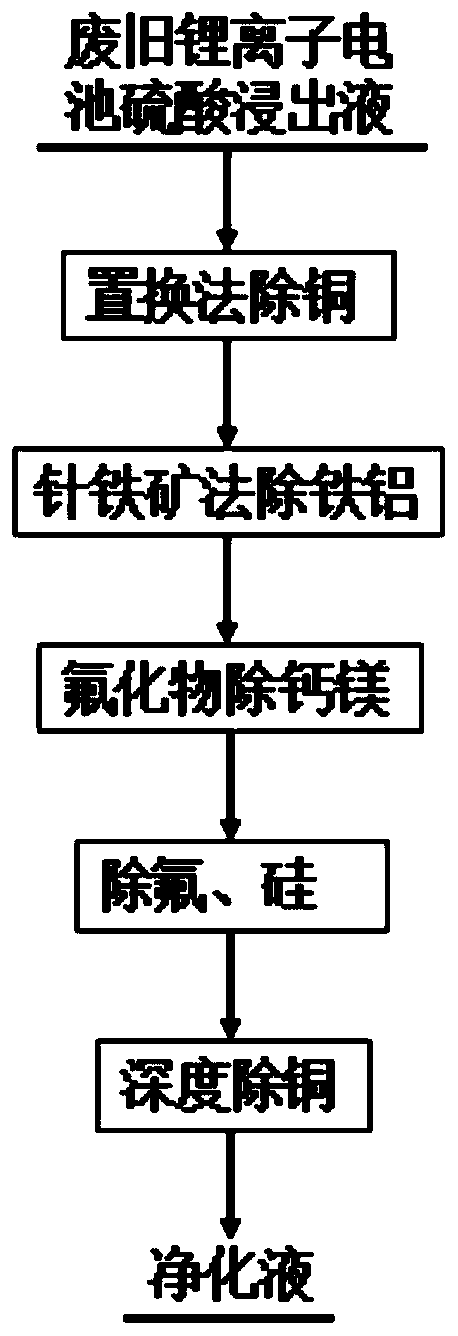
[0058] A method for removing impurities from the leachate of waste batteries, comprising the following steps:
[0059] (1) Substitution and copper removal
[0060] Take 2L of the sulfuric acid leaching solution of waste lithium-ion batteries (in which the concentration of Ni is 48.93g / L, the concentration of Co is 45.43g / L, the concentration of Mn is 18.49g / L, and the concentration of Cu is 3.65g / L). 8g of iron powder, stirred for 1 hour, filtered to obtain the copper-removed solution, and the Cu concentration in the copper-removed solution was detected to be 0.1981g / L.
[0061] (2) goethite method to remove iron and aluminum
[0062] Take 1.8L of the copper-removed liquid obtained in step (1), adjust pH=2, take samples and titrate ferrous ions, Fe 2+ The concentration is 4.20g / L, at 60°C, add 8g of 30% hydrogen peroxide, and stir for 30min, the titrated ferrous ions are all oxidized. Take 20mL ferrous removal solution and dilute it to 100mL as the base liquid, add 20% Shua...
Embodiment 2
[0070] (1) Substitution and copper removal
[0071] Take 2L of the sulfuric acid leaching solution of waste lithium-ion batteries (in which the concentration of Ni is 61.77g / L, the concentration of Co is 37.53g / L, the concentration of Mn is 3.60g / L, and the concentration of Cu is 2.22g / L). 5.5g of iron powder, stirred for 1h, filtered to obtain the copper-removed solution, and the Cu concentration in the copper-removed solution was detected to be 0.1014g / L.
[0072] (2) goethite method to remove iron and aluminum
[0073] Take 1.8L of the copper-removed liquid obtained in step (1), adjust pH=2, take samples and titrate ferrous ions, Fe 2+ The concentration is 3.75g / L. At 60°C, add 3g of sodium chlorate and stir for 30min. After titration, all ferrous ions are oxidized. Take 20mL of ferrous removal solution and dilute it into 100mL as the bottom solution, add 15% soda ash solution and the remaining ferrous solution to the bottom solution respectively by means of peristaltic p...
Embodiment 3
[0081] (1) Substitution and copper removal
[0082]Take 2L of sulfuric acid leaching solution from waste lithium-ion batteries (in which the concentration of Co is 94.52g / L, the concentration of Ni is 2.51g / L, and the concentration of Cu is 6.89g / L). The solution after copper removal was obtained by filtration, and the Cu concentration in the solution after copper removal was detected to be 0.091g / L.
[0083] (2) goethite method to remove iron and aluminum
[0084] Take 1.8L of the copper-removed liquid obtained in step (1), adjust pH=2, take samples and titrate ferrous ions, Fe 2+ The concentration is 7.08g / L. At 60°C, add 6g of sodium chlorate and stir for 30min. After titration, all ferrous ions are oxidized. Take 20mL of ferrous removal solution and dilute it into 200mL as the bottom solution, add 15% soda ash solution and the remaining ferrous solution to the bottom solution by using a peristaltic pump to control the adding speed of the ferrous solution and soda ash sol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com