Inorganic perovskite crystal preparation method and product thereof
A perovskite precursor, inorganic calcium technology, applied in crystal growth, chemical instruments and methods, single crystal growth and other directions, can solve the problems of difficulty in preparing large-sized crystals, difficult to control the preparation process, and high crystal defect density. Inhibition of the effect of perovskite crystal nucleation, high crystal growth success rate, and low defect density
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Preparation of Inorganic Perovskite Crystals
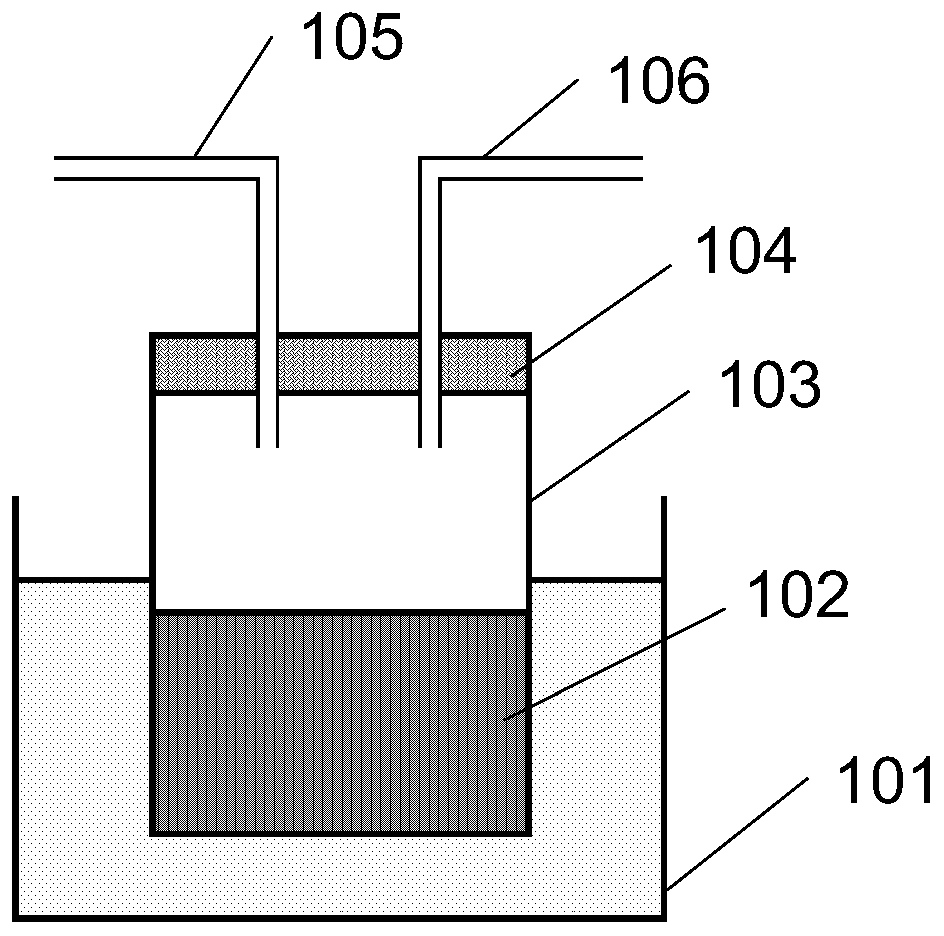
[0049] The experimental device and experimental scene for preparing inorganic perovskite are as follows: figure 1 As shown, weigh 6mmol CsBr and 6mmolPbBr 2 Add the powder into the reaction vessel 103, then add 10 mL of dimethyl sulfoxide (DMSO) using a pipette gun, cover the airtight plug 104, close the inlet and outlet 105 and the gas outlet 106, put the reaction vessel 103 into a vibrator, and vibrate Half an hour to ensure that the solute is fully dissolved to obtain the perovskite precursor solution 102, put the reaction vessel 103 filled with the perovskite precursor solution 102 into the constant temperature water bath 101, and ensure that the liquid level of the water bath is higher than that of the perovskite precursor solution 102 On the surface, the constant temperature water bath 101 was heated to 45°C and kept constant. Into the air inlet 105, a constant flow of nitrogen gas is introduced, and the flow rate o...
Embodiment 2
[0055] Preparation of Inorganic Perovskite Crystals
[0056] The experimental device and experimental scene for preparing inorganic perovskite are as follows: figure 1 As shown, weigh 4mmolCsI and 5mmolPbBr 2 Add the powder into the reaction vessel 103, then add 10 mL of dimethylformamide (DMF) using a pipette gun, cover the airtight plug 104, close the inlet and outlet 105 and the gas outlet 106, put the reaction vessel 103 into a vibrator, and vibrate Half an hour to ensure that the solute is fully dissolved to obtain the perovskite precursor solution 102, put the reaction vessel 103 filled with the perovskite precursor solution 102 into the constant temperature water bath 101, and ensure that the liquid level of the water bath is higher than that of the perovskite precursor solution 102 On the other hand, the constant temperature water bath 101 was heated to 60°C and kept constant. Into the air inlet 105, a constant flow of nitrogen gas is introduced, and the flow rate of...
Embodiment 3
[0060] Preparation of Inorganic Perovskite Crystals
[0061] The experimental device and experimental scene for preparing inorganic perovskite are as follows: figure 1 As shown, weigh 5mmol CsBr and 6mmol PbBr 2 Add the powder into the reaction vessel 103, then add 10 mL of a mixture of butyrolactone (GBL) and dimethylformamide (DMF) using a pipette gun, cover the airtight plug 104, close the inlet and outlet 105 and the gas outlet 106, Put the reaction vessel 103 into a vibrator and vibrate for half an hour to ensure that the solute is fully dissolved to obtain a perovskite precursor solution 102. Put the reaction vessel 103 filled with the perovskite precursor solution 102 into a constant temperature water bath 101 to ensure that the water bath The surface is higher than the liquid surface of the perovskite precursor solution 102, and the constant temperature water bath 101 is heated to 50° C. and kept constant. Into the air inlet 105, a constant flow of nitrogen gas is in...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com