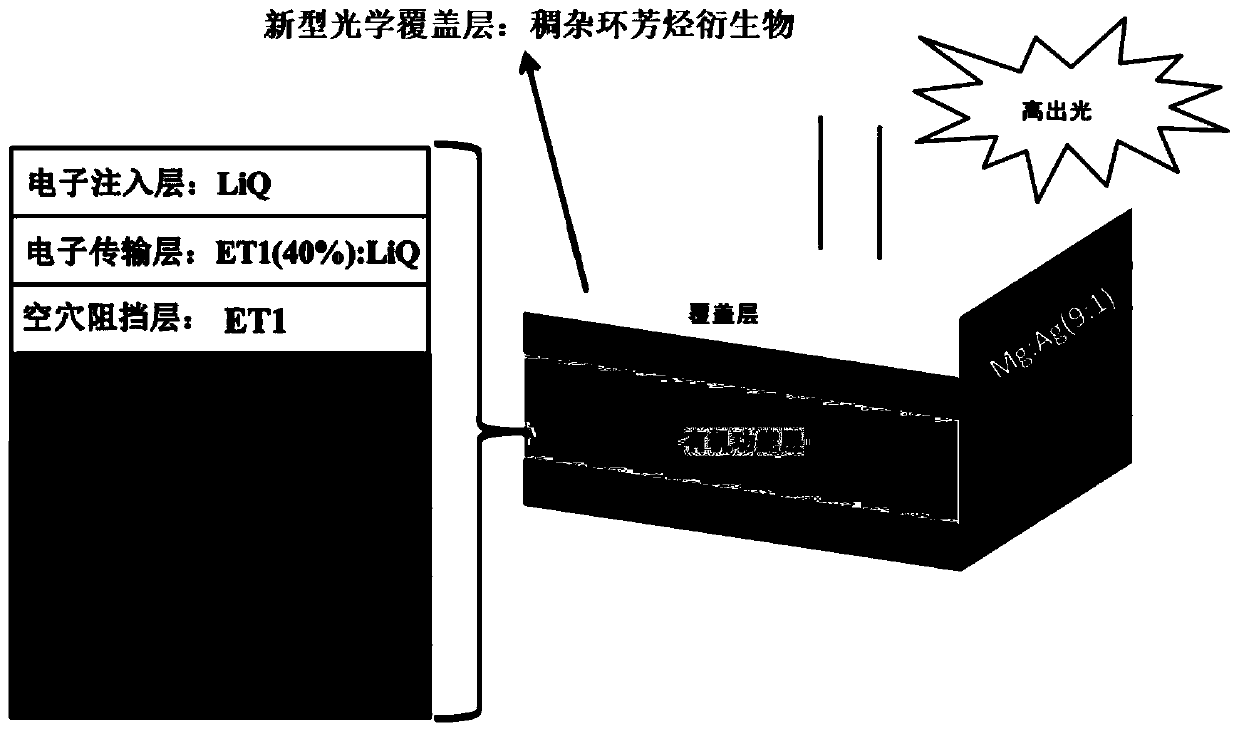
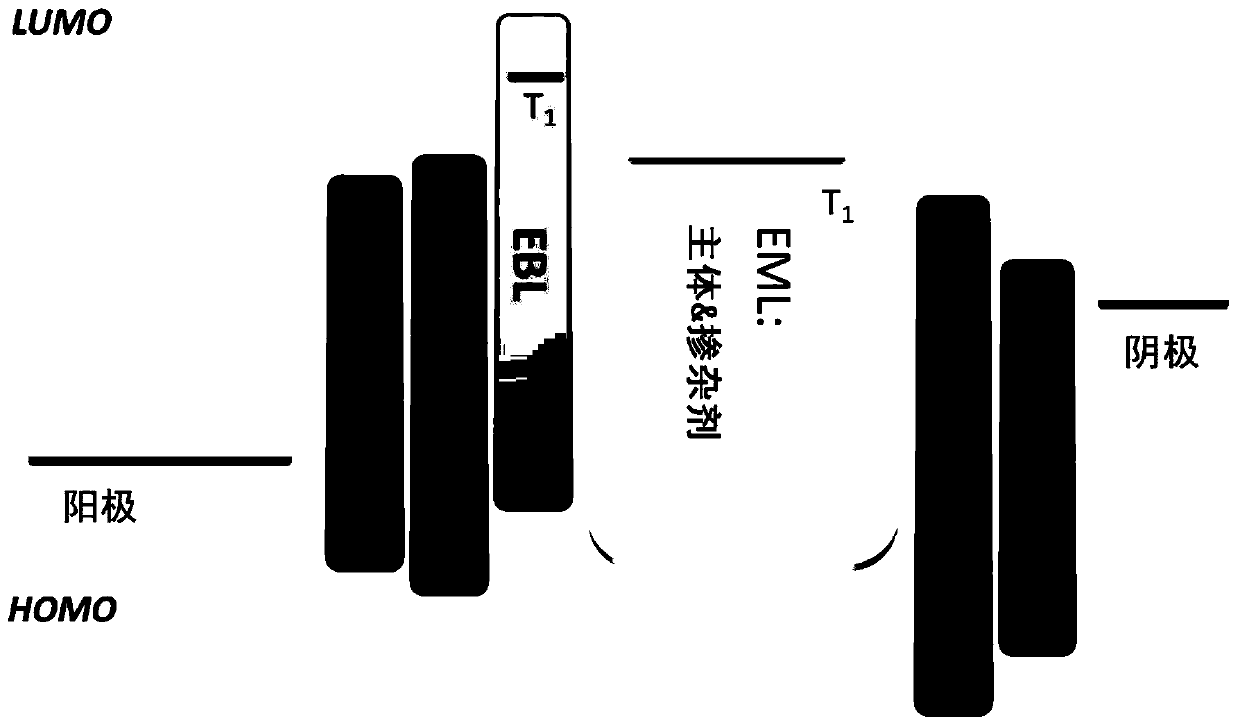
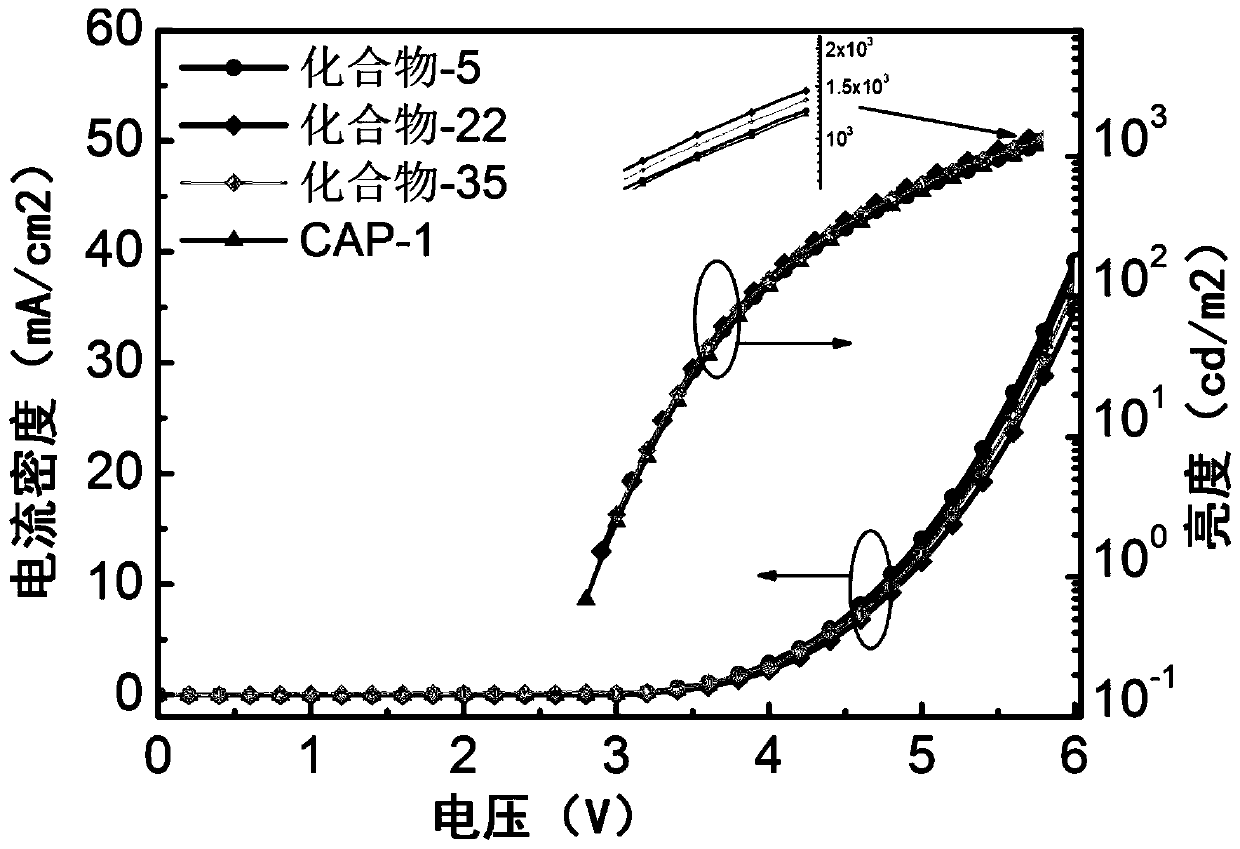
Fused heterocyclic aromatic hydrocarbon derivative as well as preparation and application thereof
A technology of heterocyclic aromatic hydrocarbons and derivatives, which is applied in the field of optoelectronic materials, can solve the problems of limiting the performance and efficiency of organic electroluminescent devices, and achieve the effects of improving light extraction efficiency, avoiding light loss, and increasing the refractive index
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0054] The present invention also provides the preparation method of described condensed heterocyclic aromatic hydrocarbon derivatives, comprising the following steps:
[0055] (1) to contain substituent R 1 , R 2 , R 3 and R 4 The boronic acid compound and the halide corresponding to the benzoheterocycle are used as raw materials, and the Suzuki reaction (Suzuki coupling reaction) occurs to obtain the substituent R 1 , R 2 , R 3 and R 4 Halides of benzoheterocycles;
[0056] (2) to contain substituent R 1 , R 2 , R 3 and R 4 The halogenated benzoheterocycle and dioxyphenothiazine are used as raw materials to undergo a Wollmann reaction (Ullmann reaction) to obtain the condensed heterocyclic aromatic hydrocarbon derivative.
[0057] In some embodiments, step (1) is specifically:
[0058] will contain the substituent R 1 , R 2 , R 3 and R 4 The substituted boronic acid compound and the halide corresponding to the benzo heterocycle are fed in a molar ratio of 1-3...
Embodiment 1
[0070] Example 1: The above compound 3 of the present invention can be synthesized by the following method.
[0071]
[0072] (1) In a 1L three-necked flask, add 2-chloro-4,6-dibromobenzothiazole (49.11g, 150mmol), phenylboronic acid (36.58g, 300mmol), potassium carbonate (41.46g, 300mmol), toluene ( 300mL), ethanol (150mL), water (150mL), ultrasonically remove the air, continue to add tetrakis (triphenylphosphine) palladium (0.52g, 0.45mmol), under the protection of nitrogen, heat up to 85 ° C and reflux for 28h, the liquid phase Monitor the completion of the reaction, cool to room temperature, wash twice with water, and beat twice with ethanol to obtain 32.82 g of 2-chloro-4,6-diphenylbenzothiazole with a yield of 68%;
[0073] (2) In a 1L three-necked flask, add 2-chloro-4,6-diphenylbenzothiazole (32.18g, 100mmol), phenothiazine (21.92g, 110mmol), potassium carbonate (41.46g, 300mmol), Tri-tert-butylphosphine tetrafluoroborate (0.58g, 2mmol), xylene (350mL), ultrasonicall...
Embodiment 2
[0076] Embodiment 2: the above compound 11 of the present invention can be synthesized by the following method
[0077]
[0078] (1) In a 1L three-necked flask, add 2-chloro-7-bromobenzothiazole (37.28g, 150mmol), phenanthrene-4-ylboronic acid (33.31g, 150mmol), potassium carbonate (41.46g, 300mmol), toluene (300mL), ethanol (150mL), water (150mL), ultrasonically remove the air, continue to add tetrakis (triphenylphosphine) palladium (0.52g, 0.45mmol), under the protection of nitrogen, the temperature was raised to 85°C and heated to reflux for 20h, the liquid After phase monitoring, the reaction was completed, cooled to room temperature, washed twice with water, and beaten twice with ethanol to obtain 36.83 g of 2-chloro-7-(phenanthrene-4-yl)benzothiazole with a yield of 71%;
[0079] (2) In a 1L three-necked flask, add 2-chloro-7-(phenanthrene-4-yl)benzothiazole (34.58g, 100mmol), phenothiazine (21.92g, 110mmol), potassium carbonate (41.46g, 300mmol ), tri-tert-butylphos...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com