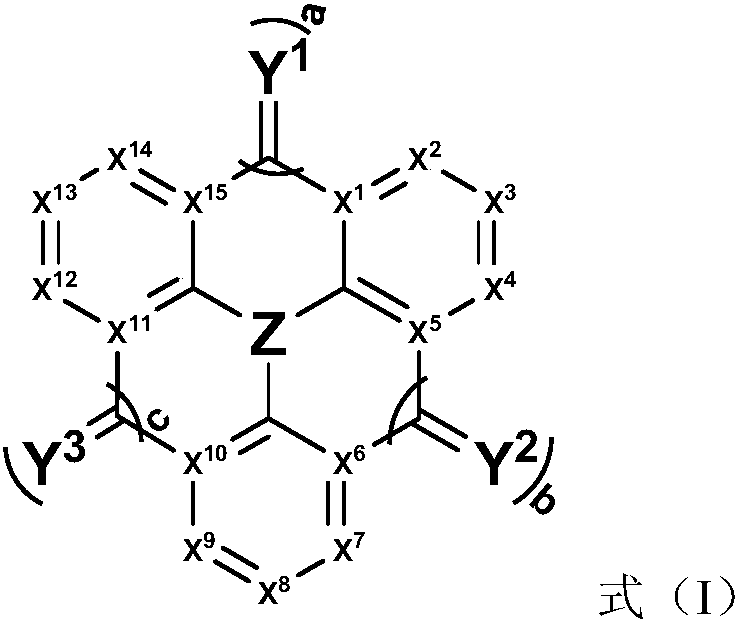
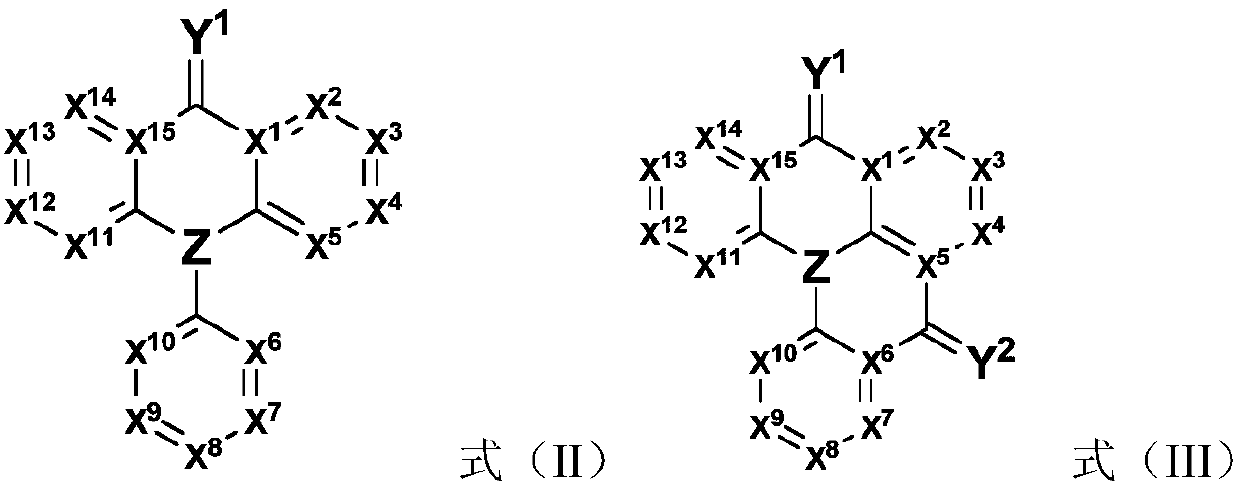
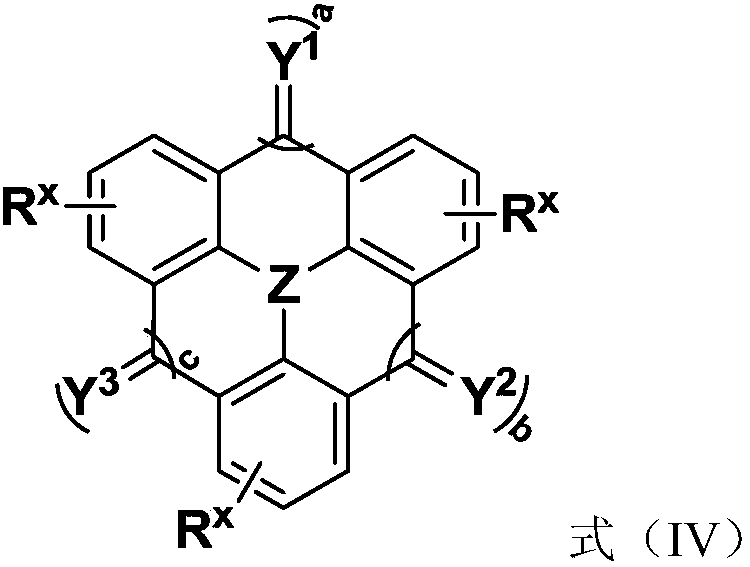
Compound, application thereof and organic light-emitting device comprising compound
A compound and selected technology, applied in the field of organic electroluminescent devices, can solve the problems that the performance needs to be further improved
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0153] Synthesis of compound M1:
[0154]
[0155] (1) Synthesis of intermediate M1-1
[0156] Add 67.5g (150mmol) of 2-bromo-1-(2-bromophenyl)-4-iodobenzene and 20.9g (100mmol) of 9,9-dimethylacridine into a freshly dried 2000mL two-necked bottle , then add 20.7g (150mmol) of anhydrous potassium carbonate, 19.0g (100mmol) of cuprous iodide, 180g (100mmol) of phenanthroline and 1200mL of dry N,N-dimethylformamide (DMF), room temperature After stirring for 0.5 hours, the temperature was raised to 130 degrees Celsius to continue the reaction for 10 hours. After the reaction was completed, it was lowered to room temperature, and the solvent in the reaction system was distilled off under reduced pressure. The crude product was dissolved in 500 mL of dichloromethane and washed with a large amount of water. The organic phase was dried with anhydrous sodium sulfate and then concentrated. Dichloromethane:petroleum ether=1:10 was used as the eluent for column chromatography to obt...
preparation example 2
[0164] Synthesis of Compound M2:
[0165] The difference from Preparation Example 1 is that 9,9-dimethylacridine was replaced by 9,9-diphenylacridine in an equivalent amount to obtain 3.2 g of product M2 with a yield of 50.8%. The molecular mass determined by mass spectrometry is: 635.21 (calculated value: 635.20); theoretical element content (%) C 44 h 30 NO 2 P: C, 83.13; H, 4.76; N, 2.20; O, 5.03; P, 4.87. Measured element content (%): C, 83.11; H, 4.75; N, 2.19.
[0166] The above analysis results indicated that the obtained product was the expected product.
preparation example 3
[0168] Synthesis of compound M10:
[0169] The difference with Preparation Example 1 is that 2-bromo-1-(2-bromophenyl)-4-iodobenzene is replaced by bis(2-bromo-4-iodophenyl)methane of equal substance amount to obtain 2.4 g product M10, the yield is 34.6%.
[0170] The molecular mass determined by mass spectrometry is: 718.30 (calculated value: 718.27); theoretical element content (%) C 49 h 39 N 2 o 2 P: C, 81.87; H, 5.47; N, 3.90; O, 4.45; P, 4.31. Measured element content (%): C, 81.89; H, 5.45; N, 3.87.
[0171] The above analysis results indicated that the obtained product was the expected product.
PUM
| Property | Measurement | Unit |
|---|---|---|
| current efficiency | aaaaa | aaaaa |
| energy conversion efficiency | aaaaa | aaaaa |
| external quantum efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com