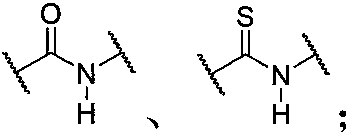
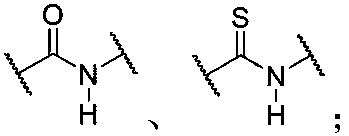
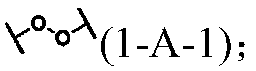Force-induced responsive supramolecular polymer
A supramolecular polymer and supramolecular technology, applied in the field of force-responsive supramolecular polymers, can solve the problems of difficult to meet the needs of multi-functional and intelligent materials, single properties and performance, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[1658] In the preparation process of the force-responsive supramolecular polymer composition, the amount of each component raw material of the force-responsive supramolecular polymer is not particularly limited, and those skilled in the art can use the actual preparation situation and the target polymer according to the actual preparation. performance tuning.
[1659] In the embodiment of the present invention, the form of the force-responsive supramolecular polymer can be solution, paste, glue, ordinary solid, elastomer, gel (including hydrogel, organogel, oligomer swelling) gel, plasticizer swollen gel, ionic liquid swollen gel), foam, etc. Among them, the content of soluble small molecular weight components contained in ordinary solid and foam materials is generally not higher than 10wt%, and the content of small molecular weight components contained in gel is generally not less than 50wt%. Ordinary solids are relatively fixed in shape and volume, have better mechanical st...
Embodiment 1
[1673] Example 1 The ureidopyrimidinone a capped with one end of a hydroxyl group is reacted with excess succinyl chloride under the catalysis of triethylamine to obtain the ureidopyrimidinone b with an acid chloride group at one end. The ureidopyrimidinone c terminated by a hydroxyl group at one end is reacted with excess succinyl chloride under the catalysis of triethylamine to obtain the ureidopyrimidinone d with an acid chloride group at one end.
[1674]
[1675] Equimolar equivalents of N-(2-hydroxyethyl)maleimide and compound d are reacted under the catalysis of triethylamine to obtain compound e. 5 molar equivalents of diol compound e containing force-sensitive groups, 9 molar equivalents of compound b and 1 molar equivalent of compound d were reacted under the catalysis of triethylamine to obtain two supramolecular monomer mixtures f. 100 parts by mass of mixture f and 5 parts by mass of compound e were fully blended, swollen in 1,4-dioxane solvent, placed in a mol...
Embodiment 2
[1676] Example 2 1 molar equivalent of hydrogen-containing dimethylsiloxane end-capped with acid chloride groups at both ends (average molecular weight of 5kDa, hydrogen content of 3%) and 2 molar equivalents of 4-hydroxypyridine-2,6-dicarboxylic acid were added to Triethylamine reacts under the catalysis of triethylamine to obtain polysiloxane a capped with force-sensitive ligand groups at both ends. The tripeptide compound glycyl-glycyl-glycine and excess 4-pentenoyl chloride are reacted under the catalysis of triethylamine to obtain the tripeptide compound b with an alkenyl group at one end. The equimolar equivalent of the obtained tripeptide compound b and ethylamine are subjected to amidation reaction to obtain compound c. Allyl hydroxyethyl ether and 5-chloromethyl-2-oxazolidinone are dissolved in toluene in a molar ratio of 1:1, potassium carbonate is used as a catalyst, and tetrabutylammonium bromide is used as a phase transfer agent to obtain Compound d with an allyl...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Elastic modulus | aaaaa | aaaaa |
| Elastic modulus | aaaaa | aaaaa |
| Elastic modulus | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com