Nano hemostatic antibacterial composite material for vascular surgery and preparation method thereof
A hemostatic antibacterial and composite material technology, which is applied in the field of hemostatic antibacterial materials for vascular surgery, can solve the problems of unclear mechanism of action, poor hemostatic effect, and single function, and achieve the goal of enriching active sites, improving adhesion, and promoting cross-linking reactions Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
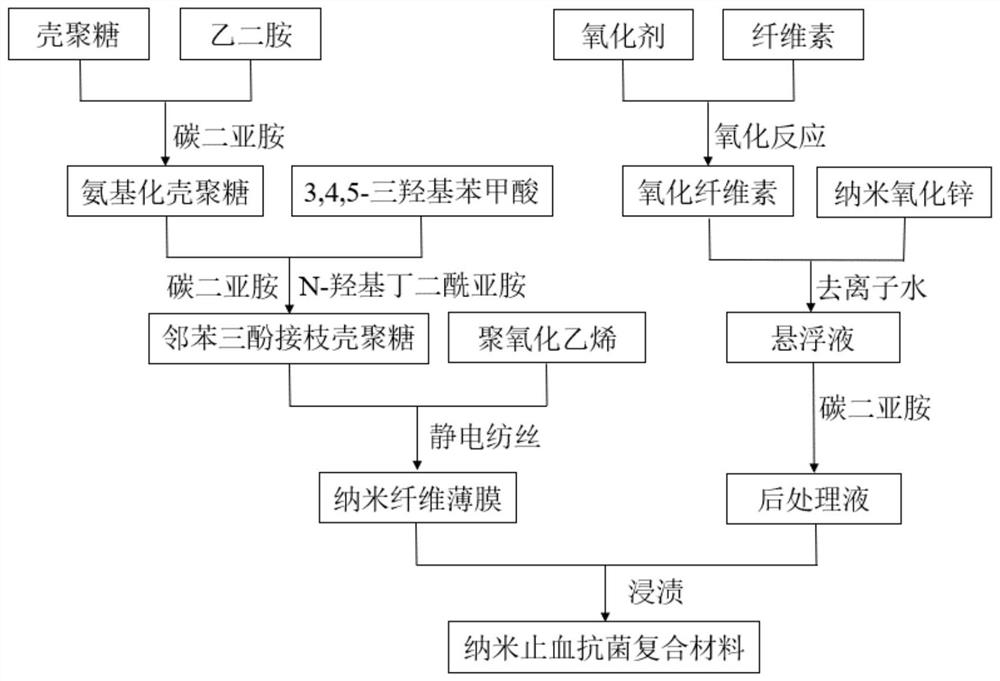
[0031] The invention provides a preparation method of a nano-hemostatic antibacterial composite material for vascular surgery, comprising the following steps:
[0032] S1, dissolving chitosan in hydrochloric acid solution, and adjusting the pH of the solution to 5.0 to 5.5 to obtain a chitosan solution; adding a predetermined amount of ethylenediamine and carbodiimide to the chitosan solution, at 35 After reacting at ~40°C for 5-7 hours, the product is sequentially subjected to dialysis and freeze-drying to obtain amino chitosan;
[0033] S2. Put the aminated chitosan solution obtained in step S1 into the hydrochloric acid solution, and adjust the pH of the solution to 5.0-5.5 to obtain the aminated chitosan solution; dissolve 3,4,5-trihydroxybenzoic acid in ethanol Obtain 3,4,5-trihydroxybenzoic acid solution; then add the 3,4,5-trihydroxybenzoic acid solution into the aminated chitosan solution to obtain a mixed solution, and add it to the mixed solution Adding a predetermi...
Embodiment 1
[0048] This embodiment provides a preparation method of a nano-hemostatic antibacterial composite material for vascular surgery, including the following steps:
[0049] S1. Dissolve 2g of chitosan in 100mL of hydrochloric acid solution with a concentration of 0.1mol / L, and adjust the pH to 5.0, then add ethylenediamine and carbodiimide in turn, and stir the reaction at 37°C for 6h with a magnetic stirrer Finally, put the reaction product into a dialysis bag with a molecular weight cut-off of 3500, and dialyze it in acidic deionized water with pH=5 for 48 hours to remove unreacted ethylenediamine and carbodiimide; then place it at -60°C Freeze-drying for 48h to obtain amino chitosan;
[0050] Wherein, the ratio of ethylenediamine to the amount of carboxyl on the chitosan carbon chain is 25:1; the ratio of carbodiimide to the amount of carboxyl on the chitosan carbon chain is 2:1.
[0051] S2. Continue dissolving the aminated chitosan obtained in step S1 in 100 mL of hydrochlor...
Embodiment 2~5
[0063] Embodiment 2~5 and comparative example 1~2
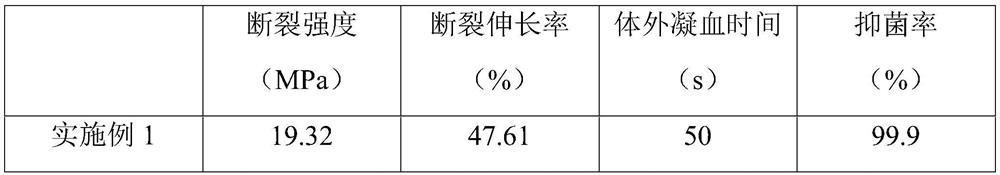
[0064] Examples 2-5 and Comparative Examples 1-2 respectively provide a preparation method of a nano-hemostatic antibacterial composite material for vascular surgery. Compared with Example 1, the difference is that the ethylenediamine and chitosan in step S1 are changed. The ratio of the amount of carboxyl on the sugar carbon chain or the ratio of the amount of carboxyl in 3,4,5-trihydroxybenzoic acid to the amount of amino in aminated chitosan in step S2, other steps are consistent with Example 1 , which will not be repeated here. Table 2 shows the proportions of specific substances corresponding to steps S1-S2 in each embodiment and comparative example.
[0065] Table 2 The ratio of the amount of substance corresponding to steps S1 to S2 of Examples 2 to 5 and Comparative Examples 1 to 2
[0066]
[0067]
[0068] Wherein, ethylenediamine was not added in Comparative Example 1, and the ratio of the amount of carboxyl ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com