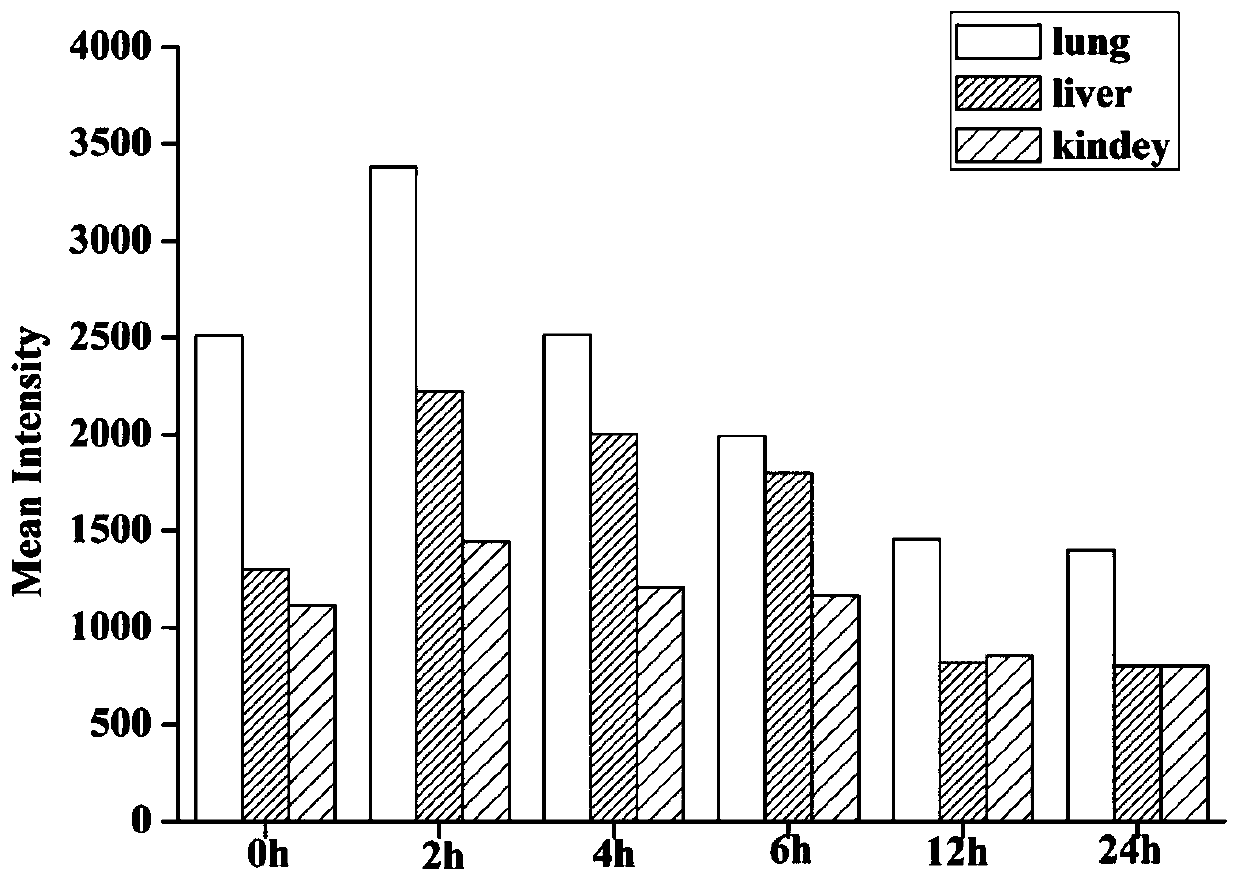
Preparation method and application of icariin sustained-release nano-gel by aerosol targeted drug delivery
A technology of icariin and nano-gel is applied in the field of medicine to achieve the effects of reducing asthmatic airway inflammatory response, good bioadhesion, and reducing the level of cytokines
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Example 1 Preparation of icariin-alginate nanogel
[0043] 1.1 Preparation of sodium alginate-water solution
[0044] At 45°C (usually 40-50°C), mix sodium alginate with water evenly to make a sodium alginate-water solution with a mass-volume concentration of 0.50% (m / v, usually 0.25-0.75%) ;
[0045] 1.2 Preparation of zinc chloride-ethanol solution
[0046] Mix zinc chloride with an ethanol solution with a concentration of 60% (usually 50-70%) by volume and vortex to prepare a chlorine concentration of 0.1% (m / v, usually 0.1-0.2%) Zinc chloride-ethanol solution;
[0047] 1.3 Preparation of icariin-zinc chloride solution
[0048] Add icariin into the zinc chloride-ethanol solution, and vortex mix, wherein, the ratio of the mass of icariin to the volume of the zinc chloride-ethanol solution is 0.3:100 (usually (0.1- 0.4): 100), that is, 0.3 g (usually 0.1-0.4 g) of icariin is added to 100 mL of zinc chloride-ethanol solution to prepare an icariin-zinc chloride solu...
Embodiment 2
[0055] Embodiment 2 Characterization of icariin-zinc alginate nanogel
[0056] 2.1 Particle size and Zeta potential test
[0057] The prepared zinc alginate nanogel solution and icariin-zinc alginate nanogel solution were diluted 10 times, and the particle size, particle size distribution and surface Zeta potential of the nanogel were measured using a nanoparticle size analyzer. The measurement was repeated 3 times, and the results were averaged, and the test results are shown in Table 1.
[0058] Table 1 Particle size and Zeta-potential (Means±SD) of zinc alginate nanogel
[0059]
[0060]
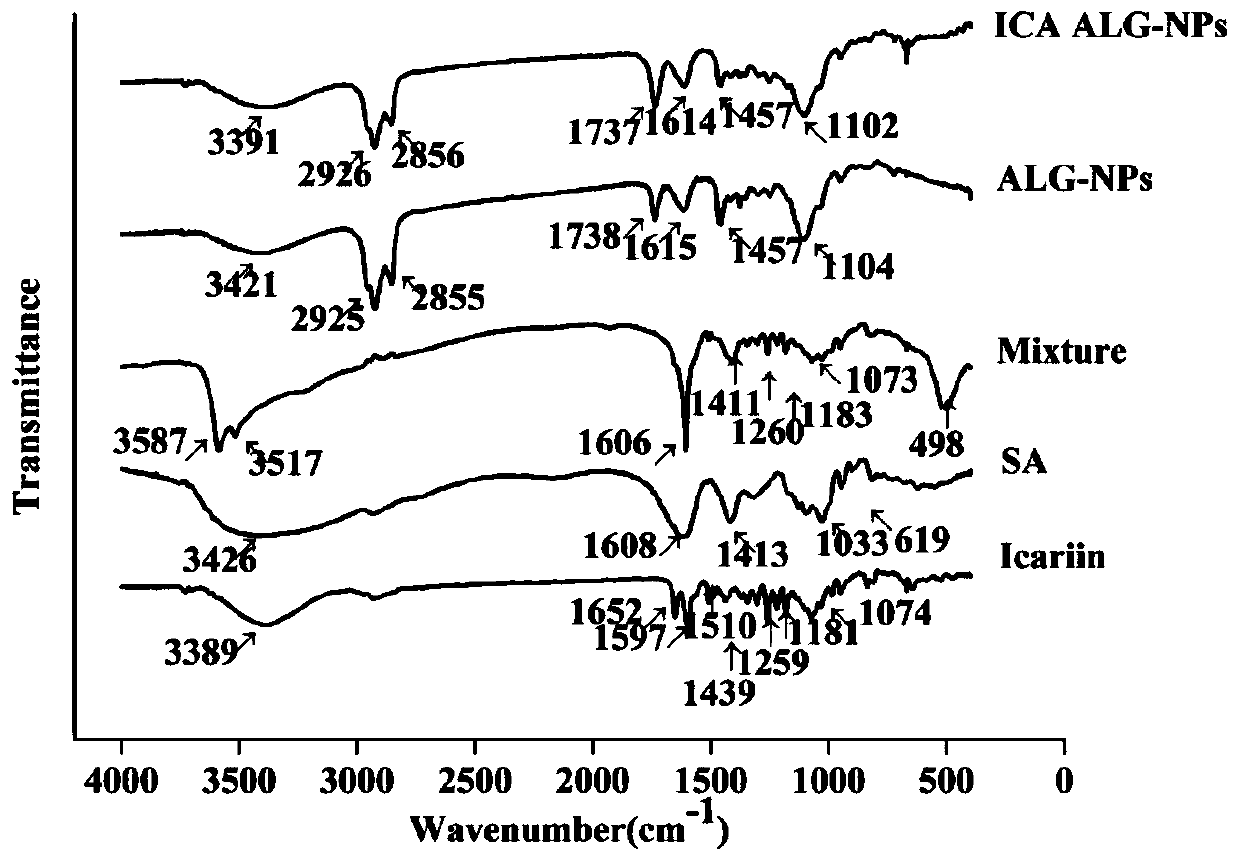
[0061] 2.2 Fourier transform infrared spectroscopy analysis
[0062] Sodium alginate, icariin, mixture of icariin-zinc chloride-sodium alginate, zinc alginate nanogel and icariin-zinc alginate nanogel dried to constant weight were mixed with bromine Potassium chloride mixed tablet, with the blank potassium bromide tablet as a control, the infrared spectrum of the sample is meas...
Embodiment 3
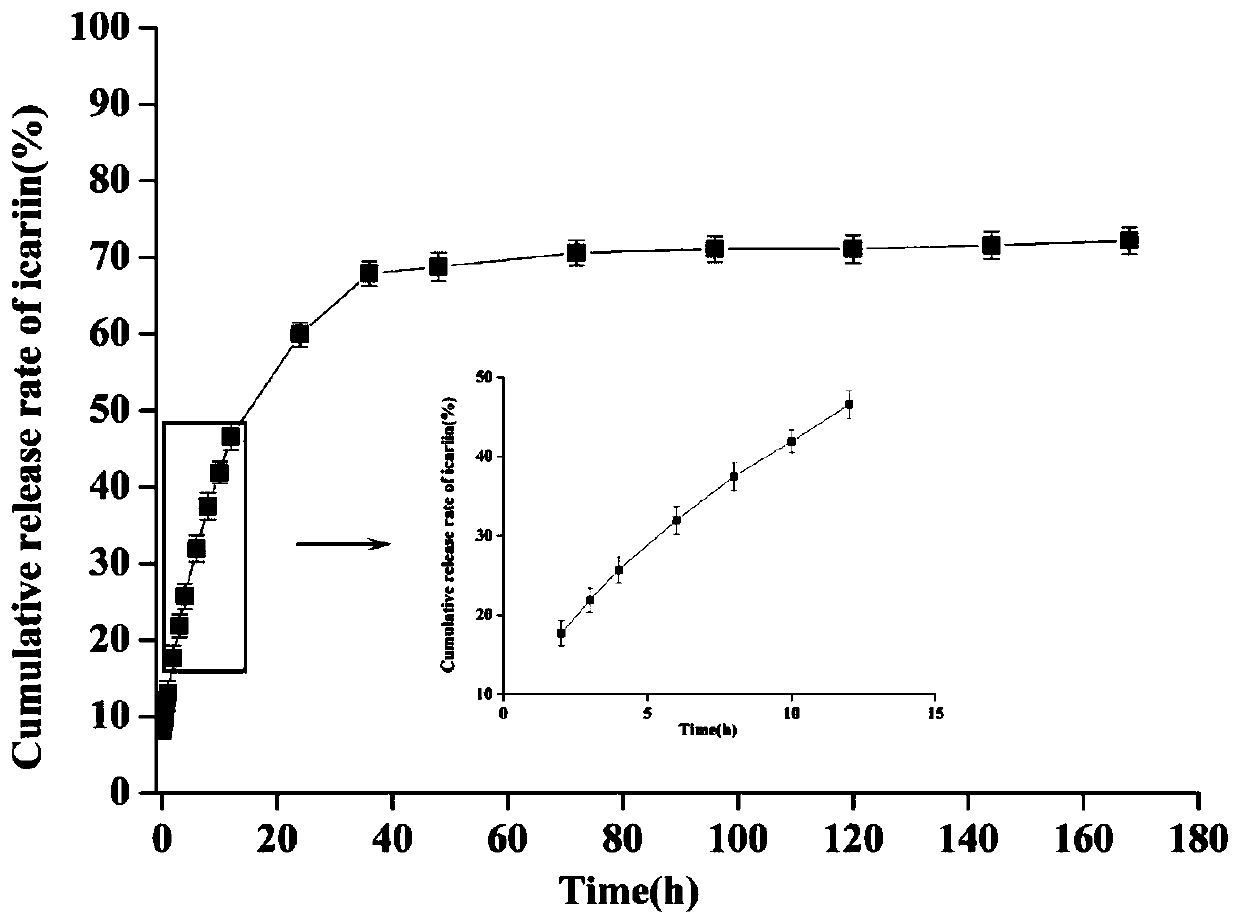
[0064] Example 3 Icariin-Zinc Alginate Nanogel Drug Loading Efficiency and Encapsulation Efficiency
[0065] Preparation of drug loading rate sample solution: Accurately weigh 3 parts of icariin-zinc alginate nanogel, 25.00 mg each, add an appropriate amount of methanol to the sample for ultrasonic extraction several times, extract icariin monomer to constant volume In a 25mL volumetric flask, after standing still, take the supernatant and filter it with a 0.45μm pore size microporous membrane, and determine the content of icariin by HPLC.
[0066] Preparation of the encapsulation efficiency sample solution: Accurately weigh 3 parts of the same batch of icariin-zinc alginate nanogel, 25.00 mg per part, add an appropriate amount of ultrapure water, and after ultrasonic treatment for 30 minutes, set the volume in a 25 mL volumetric flask. After standing still, take the supernatant and add it to the ultrafiltration tube. After centrifugation, keep the solution in the sleeve for u...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com