Diboron glycol ester as well as preparation method, intermediate and application thereof
A biboronic acid glycol ester and halogen technology, applied in the field of biboronic acid glycol ester, can solve the problems of single structure of biboronic acid glycol ester and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
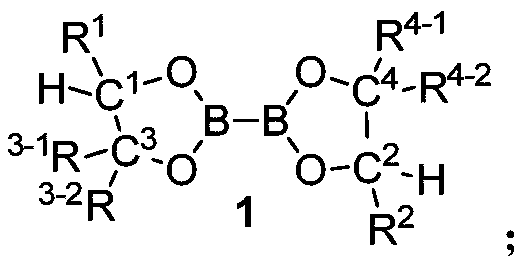
[0092] Preparation Example 1 Preparation of Chiral Diol
[0093]
[0094] Select a suitable three-necked bottle, weigh the magnesium scraps (19.5g, 0.81mol, 1.5eq) with a smooth and dry surface and replace the nitrogen three times, add re-distilled THF (200ml) and two iodine under nitrogen protection, and then add an appropriate amount of aryl Bromine reagent (total 0.54mol, 1.0eq), then heated with a hair dryer to initiate the Grignard reagent, the solution yellow faded and turned colorless, continue to add aryl bromide, keep the reaction system slightly boiling, indicating that the Grignard reaction has been initiated, and the addition of aromatic After the base bromide, the unsubstituted or substituted methyl R-mandelate (0.11mol, 0.2eq) was dissolved in re-distilled THF (50mL) solution, added dropwise to the Grignard reagent, and the reaction was heated to reflux for about 4 hours. After completion, the reaction was cooled to room temperature, quenched by pouring into s...
preparation example 2
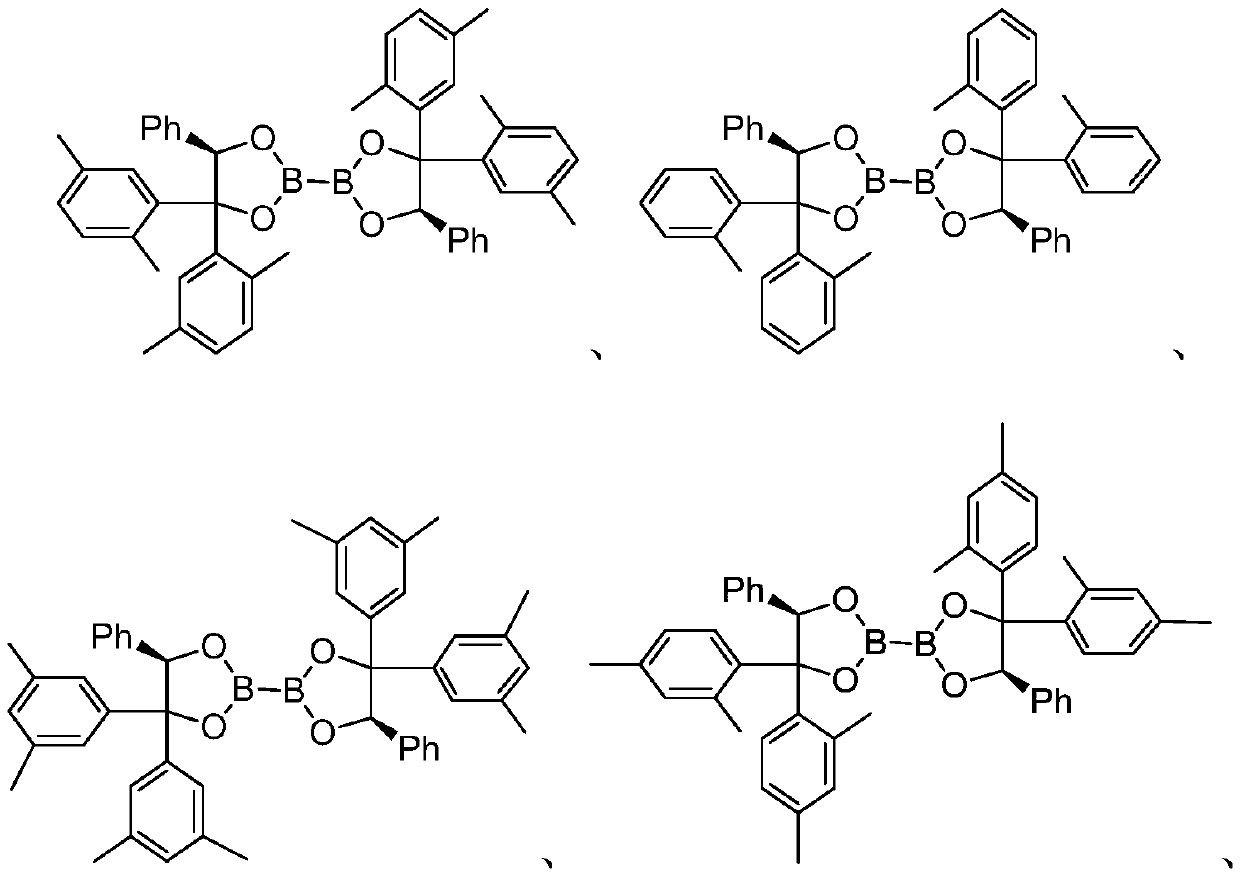
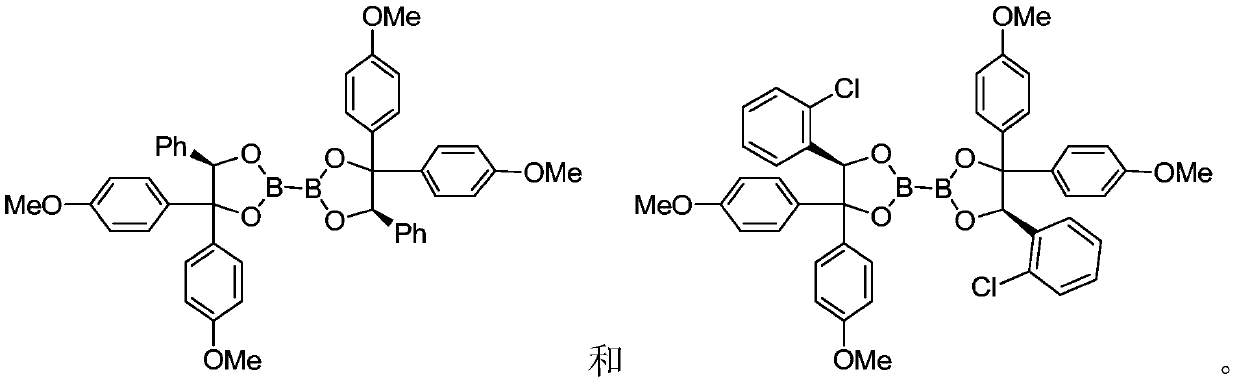
[0113] Preparation Example 2 Preparation of Chiral Diboron DB4~DB7
[0114]
[0115] Select a suitable Schlenk tube, add diol 5 (30g, 86mmol, 2.0eq), tetrahydroxydiboron (3.9g, 43mmol, 1.0eq) and an appropriate amount of 4A molecular sieves, pump nitrogen three times, add redistilled THF under nitrogen protection (150ml), the system was placed in a 70°C oil bath to reflux, and the reaction was completed in about 4 hours. The reaction system was cooled to room temperature, filtered through diatomaceous earth, washed with dichloromethane, the filtrate was spin-dried, washed with petroleum ether, and filtered again. The white solid powder of the filter cake was 28.7 g of the double boron product, and the yield was 94%. (DB4).
[0116]
[0117] White solid; 94% yield; Optical rotation: [α] D 29 =275.2°[c=1.10, CHCl 3 ]. 1 H NMR (400MHz, CDCl 3 ): δ7.70(s, 2H), 7.26(s, 2H), 7.07-6.99(m, 14H), 6.73(m, 2H), 6.59(m, 4H), 2.45(s, 6H), 2.14( s,6H),1.92(s,6H),1.53(s,6H). 13...
Embodiment 1
[0130] Example 1 Asymmetric reduction of aromatic imines to conjugate substrates
[0131]
[0132] In a clean and dry shlenk tube, aryl aldehyde (0.2 mmol, 1.0 eq) was added, the system was purged with nitrogen three times, and under nitrogen protection, ammonia methanol solution (7.0 M, 3.0 mmol, 15.0 eq) was added, and The solvent was evaporated to tetrahydrofuran (2 mL), the system was sealed, the reaction was stirred at room temperature for 1 h, under nitrogen protection, DB4 (0.11 mmol, 0.55 eq) was added, and the mixture was stirred at room temperature (20-30 °C) for 12-24 h. The system was rotary evaporated to remove excess ammonia, 1 mL of methanol was added, hydrochloric acid (3.0 M, 1 mL) was added to adjust the pH to about 3, washed with dichloromethane, the organic phase was concentrated, and the chiral diol diol5 (95% recovery) was recovered by beating with n-hexane. Rate). The aqueous phase was added with sodium hydroxide solution to adjust the pH to about 12...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com