Anti-infection silicon dioxide biological tissue adhesive and application thereof
A biological tissue, mesoporous silica technology, applied in the field of biomedicine, can solve the problems of toxic and side effects antibiotics, cross-infection, high incidence of infection, and achieve the effect of high activity and improved adhesion effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0042] The preparation of example 1 mesoporous silica
[0043] sample 1
[0044]Weigh 0.3g CTAB into a 500mL three-necked bottle, add 96mL of distilled water, protect the inside of the reaction device with nitrogen gas, and stir it magnetically at 70°C for 1 hour to fully dissolve it. Measure 45mL of n-octane at a rate of 2 drops / second Drop into the above CTAB solution system. After dropping, continue to stir for 20 minutes to form a uniform emulsion. Measure 3.2 mL tetraethyl orthosilicate TEOS and drop it into the above solution at a rate of 2 drops / second. After dropping, weigh 0.066g of lysine and add to the above solution. Then measure 8.9 mL of methyl methacrylate monomer and drop it into the above solution at a rate of 2 drops / second. After the dropwise addition, 113.4 mg of azobisisobutylamidine hydrochloride AIBA was weighed and added to the reaction solution. After the addition was complete, the reaction was continued for 4 hours under magnetic stirring in a wa...
example 2
[0055] Example 2 Preparation of lysozyme-loaded mesoporous silica nanoparticles
[0056] Weigh 40 mg of lysozyme into a 10 mL vial, add 8 mL of PBS solution with pH 6.8, and stir magnetically at 200 rpm for 1 hour at 4°C to fully dissolve the lysozyme. Then, 20 mg of the mesoporous silica nanoparticles prepared in Example 1 were weighed and put into the above-mentioned lysozyme solution, and magnetically stirred at 200 rpm for 3 hours at 4°C. After stirring, centrifuge at 8000 rpm for 3 minutes, remove the supernatant, and obtain mesoporous silica nanoparticles loaded with lysozyme protein.
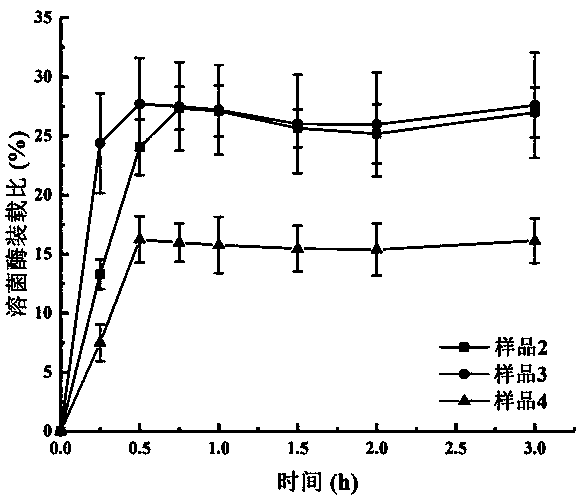
[0057] During the preparation process of the lysozyme-loaded mesoporous silica nanoparticles, samples were taken at 15 minutes, 30 minutes, 45 minutes, 1 hour, 1.5 hours, 2 hours, and 3 hours. The sampling method is as follows: absorb 500 μL of the stirring solution and centrifuge at 8000 rpm for 3 minutes, then take 200 μL of the supernatant and store it at 4° C. for testing. Prepare C...
example 3
[0060] Example 3 Carrying Lysozyme Mesoporous Silica Nanoparticle Drug Release Assay
[0061] Centrifuge the remaining solution at 8000rpm for 3 minutes after loading lysozyme in Example 2 for 3 minutes, transfer the solid precipitate to a 50mL flat-bottomed flask, add 25mL of pH6.8 phosphate buffer, stir magnetically in a 37°C water bath, and release medicine 24 hours. Samples were taken at 15 minutes, 30 minutes, 1 hour, 1.5 hours, 2 hours, 3 hours, 4 hours, and 5 hours. The sampling method is as follows: absorb 1000 μL of the stirring solution and centrifuge at 8000 rpm for 3 minutes, then take 500 μL of the supernatant and store it at 4°C for testing, add 500 μL of PBS with pH 6.8 to the remaining liquid and solid, blow well, and add back to release the drug system.
[0062] Mix and shake the 500 μL supernatant obtained at each time point with the Coomassie Brilliant Blue staining solution described in Example 2, and after dark staining for 3 minutes, test the UV-Vis abs...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com