Synthesis method of alcohol compound
A synthesis method and technology for ketone compounds, applied in the field of catalysis, can solve the problems of high price and cost of precious metal catalysts, strict requirements on raw material impurities, and easy occurrence of side reactions, etc. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0052] The present invention provides a method for synthesizing ketone compounds, comprising: using a carbon-coated nickel nanocomposite material containing alkaline earth metals as a catalyst, and catalyzing the ketone compounds to carry out a hydrogenation reduction reaction in a hydrogen atmosphere; the chemical reaction equation is exemplified as follows:
[0053]
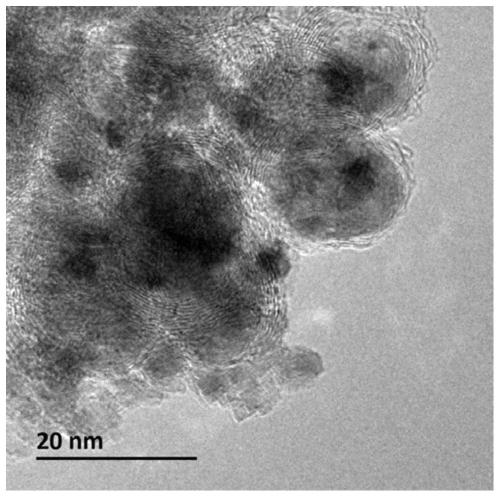
[0054] Wherein, the nanocomposite material has a core-shell structure with a shell layer and an inner core, the shell layer is a graphitized carbon layer containing alkaline earth metal, nitrogen and oxygen, and the inner core is nickel nanoparticles.
[0055] In some embodiments, the ketone compound can be aliphatic ketone, aryl ketone or alicyclic ketone, namely R 1 and R 2 Can be alkyl, cycloalkyl, aryl and R 1 and R 2 Can be the same or different, wherein, aliphatic ketone refers to a ketone in which carbon atoms in the molecule are connected into a chain, which is open-chain; attached to the aromatic...
preparation example 1
[0094] (1) Weigh 10g of nickel acetate, 10g of citric acid, and 20g of hexamethylenetetramine, add them to a beaker containing 30mL of deionized water, stir at 70°C to obtain a homogeneous solution, and continue heating and evaporating to dryness Obtain a solid precursor. Tests have proved that the solid precursor obtained in this step is soluble in water.
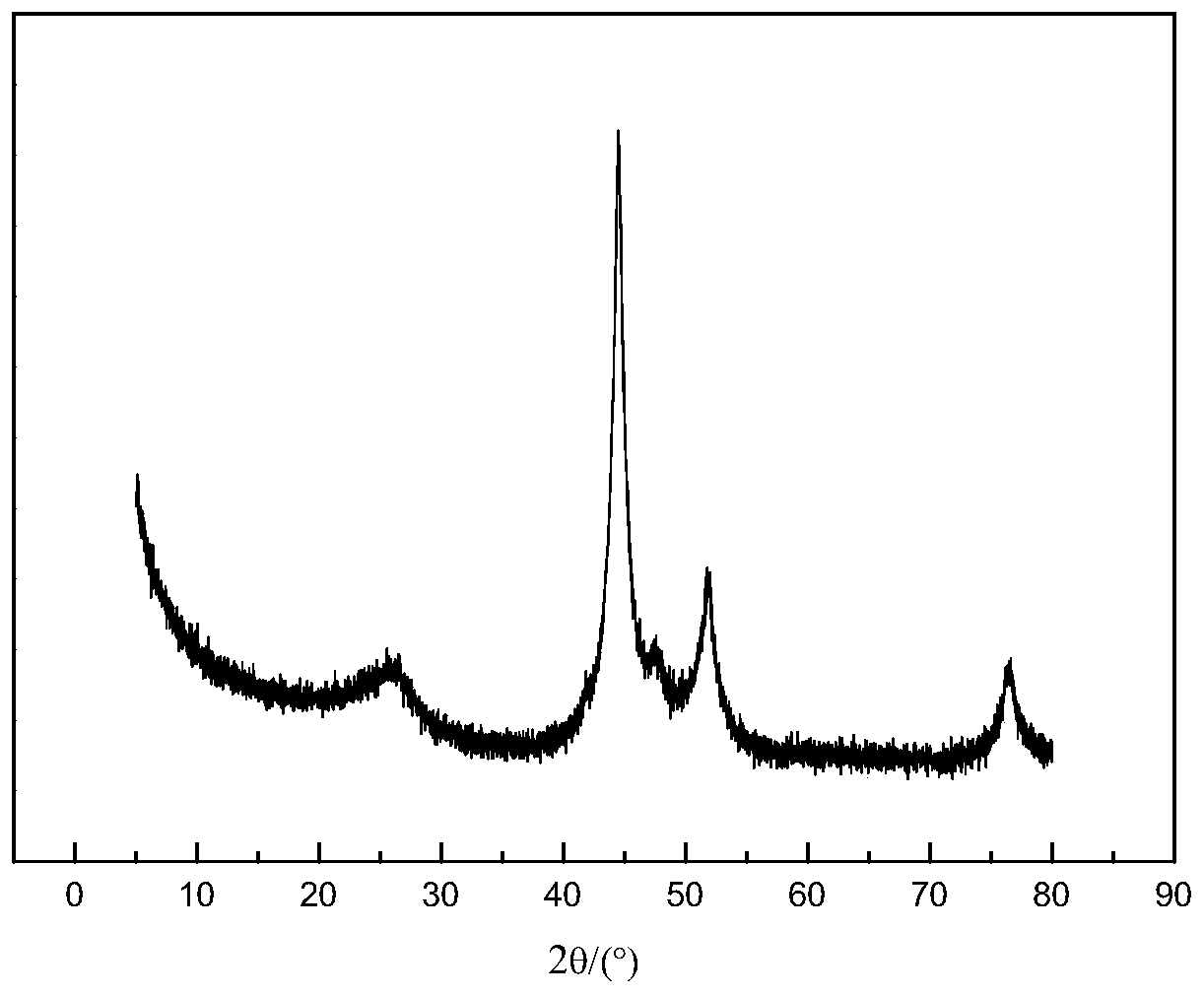
[0095] (2) Place the solid precursor obtained in step (1) in a porcelain boat, then place the porcelain boat in the constant temperature zone of the tube furnace, feed nitrogen gas with a flow rate of 100mL / min, and set the temperature at a rate of 5°C / min. Raise the temperature to 650°C, stop the heating after keeping the temperature for 2 hours, and cool to room temperature under a nitrogen atmosphere to obtain a carbon-coated nickel material.
[0096] (3) Weigh 2g of the carbon-coated nickel material obtained in step (2), add 4mL of an aqueous solution containing 0.5128g of magnesium nitrate, soak for 24h, and then dry...
preparation example 2
[0103] (1) Weigh 10g of nickel acetate, 20g of citric acid, and 20g of hexamethylenetetramine, add them into a beaker containing 100mL of deionized water, stir at 80°C to obtain a homogeneous solution, and continue heating and evaporating to dryness to obtain solid precursor.
[0104] (2) Place the solid precursor obtained in step (1) in a porcelain boat, then place the porcelain boat in the constant temperature zone of the tube furnace, feed in nitrogen gas with a flow rate of 150mL / min, and heat at a rate of 5°C / min. The temperature was raised to 600°C, the temperature was kept constant for 2 hours, the heating was stopped, and the carbon-coated nickel material was obtained by cooling to room temperature under a nitrogen atmosphere.
[0105] (3) Weigh 2 g of the carbon-coated nickel material obtained in step (2), add 4 mL of an aqueous magnesium nitrate solution containing 0.8589 g, impregnate for 24 h, and then dry at 120° C.
[0106] (4) The dried material obtained in ste...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mesopore volume | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com