Synthetic method of amino-anisole compound
An aminoanisole and a synthesis method technology, applied in the field of catalysis, can solve the problems of easy agglomeration, great environmental damage, easy to be eluted, etc., and achieve the effects of being suitable for storage and transportation, good catalytic performance, and good repeatability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0055] The invention provides a method for synthesizing aminoanisole compounds, comprising: using carbon-coated nickel nanocomposites containing alkaline earth metals as a catalyst, and catalyzing nitroanisole compounds for hydrogenation reduction reaction in a hydrogen atmosphere ; The chemical reaction equation is illustrated as follows,
[0056]
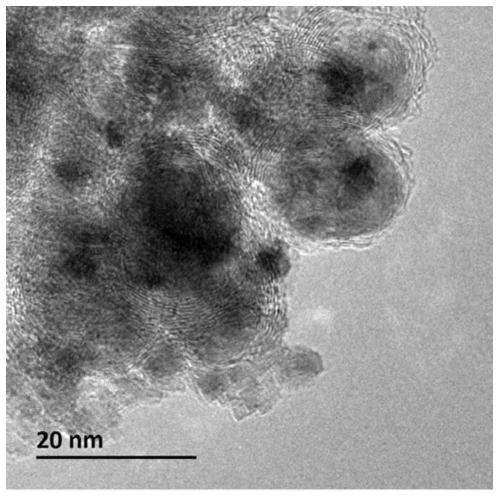
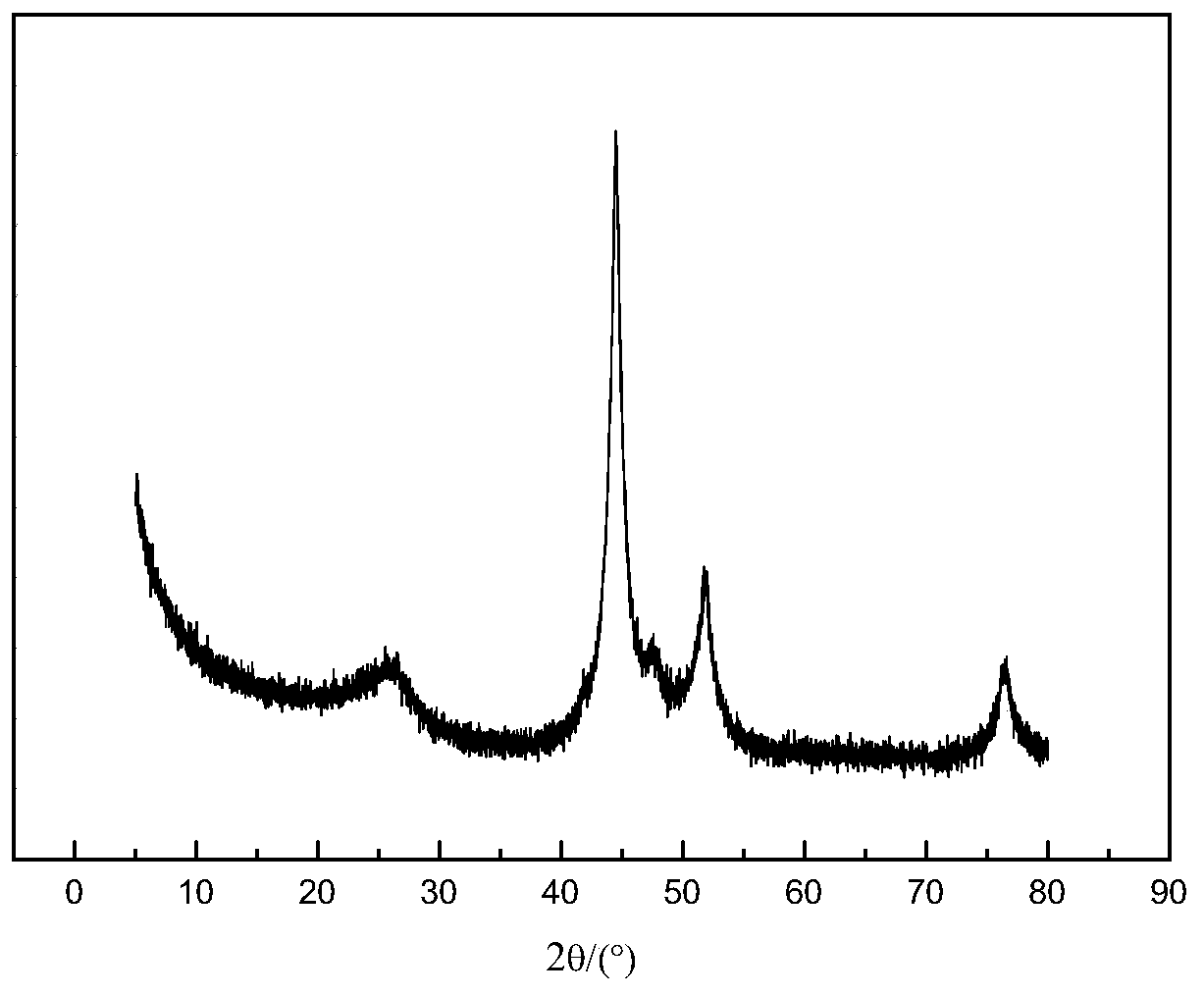
[0057] Wherein, the nanocomposite material has a core-shell structure with a shell and an inner core, the shell is a graphitized carbon layer containing alkaline earth metals, nitrogen and oxygen, and the inner core is nickel nanoparticles.
[0058] In some embodiments, the benzene ring of the nitroanisole compound also contains a substituent, and the substituent R is selected from C 1-20 One or more of the alkyl, cycloalkyl and aryl groups, the above-mentioned substitutions can be mono-substituted or multi-substituted, such as p-nitroanisole, m-nitroanisole or 3-methyl -4-Nitroanisole.
[0059] In some embodiments, the catal...
preparation example 1
[0095] (1) Weigh 10g of nickel acetate, 10g of citric acid, and 20g of hexamethylenetetramine, add them to a beaker containing 30mL of deionized water, stir at 70°C to obtain a homogeneous solution, and continue heating and evaporating to dryness Obtain a solid precursor. Tests have proved that the solid precursor obtained in this step is soluble in water.
[0096] (2) Place the solid precursor obtained in step (1) in a porcelain boat, then place the porcelain boat in the constant temperature zone of the tube furnace, feed nitrogen gas with a flow rate of 100mL / min, and set the temperature at a rate of 5°C / min. Raise the temperature to 650°C, stop the heating after keeping the temperature for 2 hours, and cool to room temperature under a nitrogen atmosphere to obtain a carbon-coated nickel material.
[0097] (3) Weigh 2g of the carbon-coated nickel material obtained in step (2), add 4mL of an aqueous solution containing 0.5128g of magnesium nitrate, soak for 24h, and then dry...
preparation example 2
[0104] (1) Weigh 10g of nickel acetate, 20g of citric acid, and 20g of hexamethylenetetramine, add them into a beaker containing 100mL of deionized water, stir at 80°C to obtain a homogeneous solution, and continue heating and evaporating to dryness to obtain solid precursor.
[0105] (2) Place the solid precursor obtained in step (1) in a porcelain boat, then place the porcelain boat in the constant temperature zone of the tube furnace, feed in nitrogen gas with a flow rate of 150mL / min, and heat at a rate of 5°C / min. The temperature was raised to 600°C, the temperature was kept constant for 2 hours, the heating was stopped, and the carbon-coated nickel material was obtained by cooling to room temperature under a nitrogen atmosphere.
[0106] (3) Weigh 2 g of the carbon-coated nickel material obtained in step (2), add 4 mL of an aqueous magnesium nitrate solution containing 0.8589 g, impregnate for 24 h, and then dry at 120° C.
[0107] (4) The dried material obtained in ste...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mesopore volume | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com