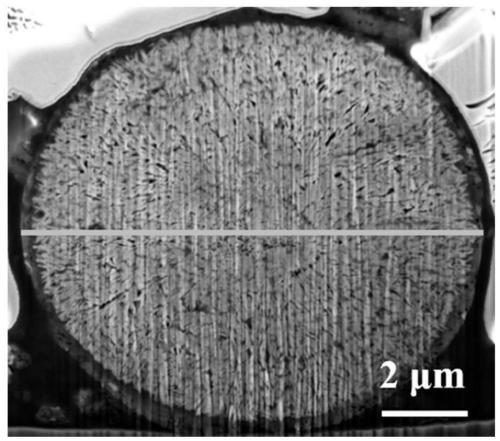
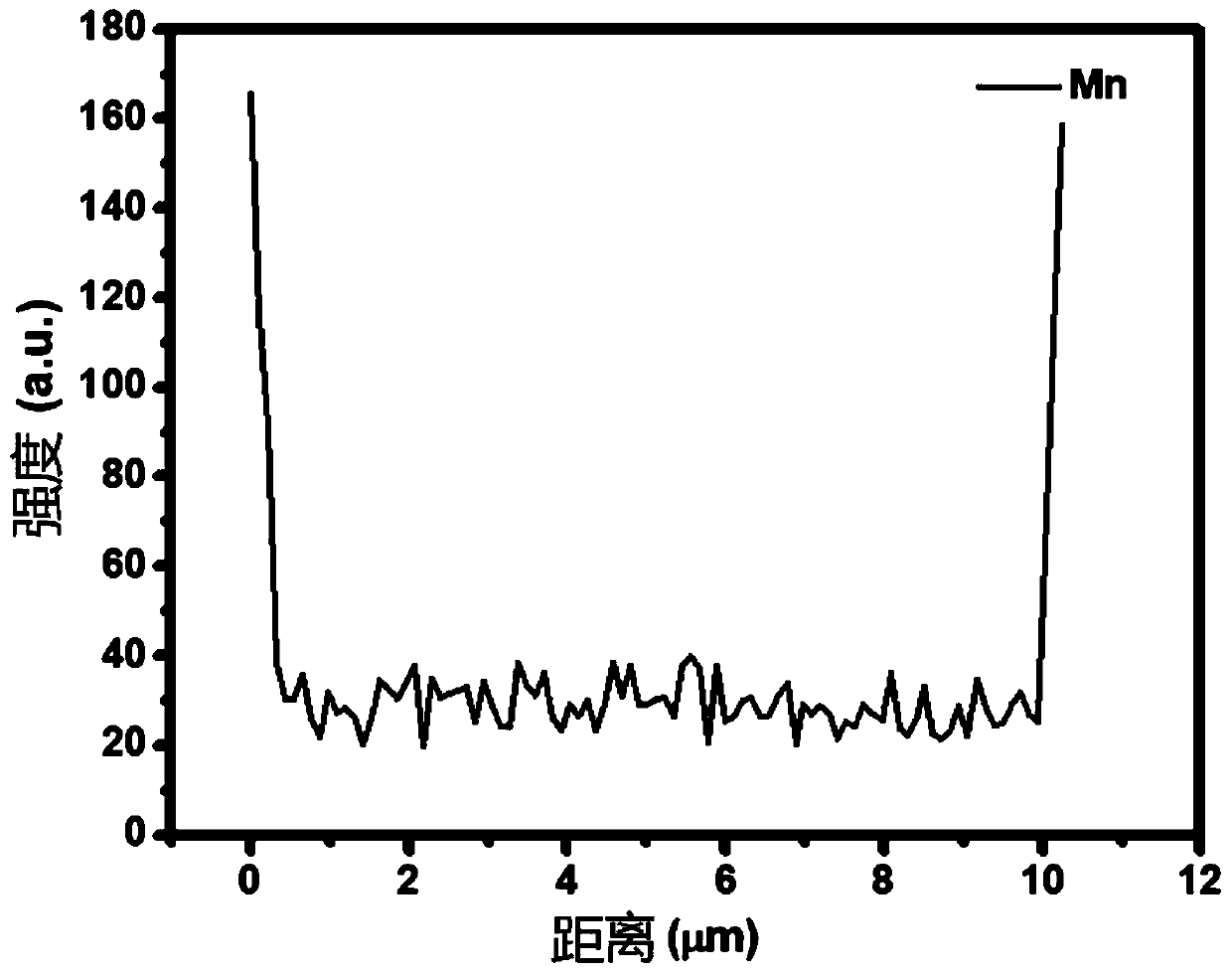
Modification method of high-nickel ternary anode material of lithium ion battery
A high-nickel ternary material, lithium-ion battery technology, applied in battery electrodes, positive electrodes, electrical components, etc., can solve problems such as complex methods, and achieve the effects of reducing breakage, reducing cracks, and improving cycle stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] (1) Commercialized LiNi 0.8 co 0.15 al 0.05 o 2 The precursor and lithium hydroxide were mixed and ground at a molar ratio of 1:1.05, and sintered in a muffle furnace from room temperature to 480 °C in an air atmosphere for 5 hours at a heating rate of 5 °C / min to obtain a lithiated precursor of NCA.
[0041] (2) Mix and dissolve a certain amount of manganese acetate, lithium hydroxide and citric acid into an appropriate amount of deionized water, stir for 30 minutes to dissolve completely, wherein the molar ratio of manganese acetate, lithium hydroxide and anhydrous citric acid is 1:1 :1. The resulting 20g mixed solution (0.3g of manganese acetate) was heated to 80°C, and then 2.05g of the lithiated precursor of NCA obtained in step (1) was added to it to obtain a precursor of coated nanoparticles Li-Mn-O , wherein, Mn is 3mol% of NCA, continue to stir for 5 hours and evaporate to dryness.
[0042] (3) Sinter the precursor of coated nanoparticle Li-Mn-O in a tube ...
Embodiment 2
[0045] (1) Commercialized LiNi 0.8 co 0.15 al 0.05 o 2 The precursor and lithium hydroxide were mixed and ground at a molar ratio of 1:1.03, and then sintered in a muffle furnace from room temperature to 480°C for 5 hours in an air atmosphere at a heating rate of 5°C / min to obtain LiNi 0.8 co 0.15 al 0.05 o 2 Lithiated precursors.
[0046] (2) Mix and dissolve a certain amount of manganese acetate, lithium hydroxide and citric acid into an appropriate amount of deionized water, stir for 30 minutes to dissolve completely, wherein the molar ratio of manganese acetate, lithium hydroxide and anhydrous citric acid is 2:2 :1. The resulting 20g mixed solution (0.41g of manganese acetate) was heated to 80°C, and then 2.02g of the LiNi obtained in step (1) was added thereto 0.8 co 0.15 al 0.05 o 2 The precursor was lithiated to obtain a precursor coated with Li-Mn-O nanoparticles, wherein Mn was 5 mol% of NCA, and the stirring was continued for 6 hours to evaporate the water...
Embodiment 3
[0051] (1) Commercialized LiNi 0.8 co 0.15 al 0.05 o 2 The precursor and lithium hydroxide were mixed and ground at a molar ratio of 1:1.05, and then sintered in a muffle furnace from room temperature to 480°C for 5 hours in an air atmosphere at a heating rate of 5°C / min to obtain LiNi 0.8 co 0.15 al 0.05 o 2 lithiated precursors.
[0052] (2) Mix and dissolve a certain amount of manganese acetate, lithium hydroxide and citric acid into an appropriate amount of deionized water, stir for 30 minutes to dissolve completely, wherein the molar ratio of manganese acetate, lithium hydroxide and citric acid is 1:1:1 , add ammonia water dropwise, the obtained mixed solution of 20g (1.01g of manganese acetate) is heated to 80°C, and then 1.96g of LiNi obtained in step (1) is added thereto 0.8 co 0.15 al 0.05 o 2 The lithiated precursor was obtained to obtain a precursor coated with nanoparticles Li-Mn-O, wherein Mn was 10 mol% of NCA, and the stirring was continued for 8 hours...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com