Polyethylene glycol modified recombinant human basic fibroblast growth factor
A fibroblast and growth factor technology, applied in the direction of fibroblast growth factor, growth factor/inducer, drug combination, etc., can solve problems such as unexplained modification sites, resistance to protease degradation, activity, apparent molecular weight, etc. , to achieve the effect of easy separation and purification and high uniformity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Example 1 Preparation of recombinant human basic fibroblast growth factor
[0050] 1) Construction of high-efficiency expression strains:
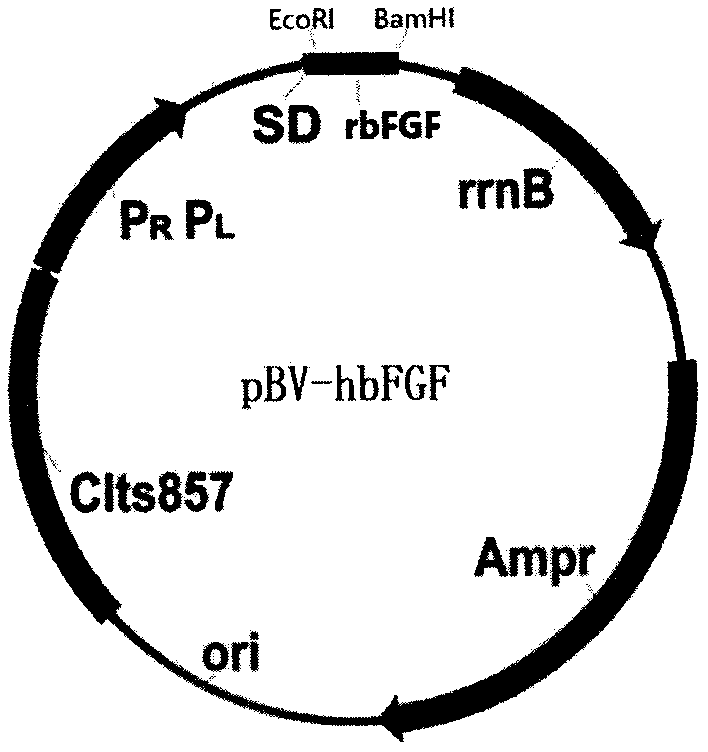
[0051] Refer to the method provided in the article "Cloning and Expression of Human Basic Fibroblast Growth Factor Gene in Escherichia coli" in the Chinese Journal of Biochemical Pharmaceutics, 1996, issue, clone the DNA sequence shown in SEQ ID NO.1 into the pBV220 vector Construct a high-efficiency expression vector pBV-hbFGF, the structure is as follows figure 1 Shown. The expression vector was transformed into Escherichia coli DH5α, and a high-efficiency expression host strain was established. See the amino acid sequence and coding DNA sequence of recombinant human bFGF image 3 .
[0052] The seed medium used ordinary LB medium. The fermentation medium adopts M9 medium plus casein hydrolysate, the formula is as follows:
[0053] Reagent name Content (g / L) Na 2 HPO 4 ·12H 2 O
12.0 KH 2 PO 4
3.0 NH 4 Cl
1.0 NaCl0.5 Peptone5....
Embodiment 2
[0059] Example 2. PEGylation reaction
[0060] Reaction conditions: pH 5.0 (usually 4.0-6.0 is optional), 4 degrees Celsius (usually 2-8°C is optional), rhbFGF concentration is 5 mg / ml (the optional range is usually 2-10 mg / ml).
[0061] Steps: Take the bFGF concentrate obtained in Example 1, add 3 times the moles of bFGF (usually 2-10 times) of excess mPEG20kDa-ALD, shake until the PEG is completely dissolved; add NaCNBH 3 To the concentration of 20mM / L (usually 10-100mM) and quickly shake to dissolve; 2-8 ℃ reaction for 15 hours (usually 5-20 hours is optional); add glycine 2 times the amount of PEG to terminate the reaction . The reaction product can be represented by the following formula:
[0062]
Embodiment 3
[0063] Example 3. Purification of reaction products
[0064] 1) Molecular sieve chromatography
[0065] A 5.6cm×100cm Sephacryl S-200 chromatographic column is used, and the balance buffer is a weak acid solution, namely 20mM NaNc-HAc, pH4.0. Add 0.1M NaCl to increase ionic strength. The loading volume is 2-5% of the column volume, and the flow rate is 30ml / min. Peristaltic pump, model 100, product of Baoding Lange Constant Flow Pump Co., Ltd.; UV detector, model 8823B, Beijing Binda Yingchuang Technology Co., Ltd.;
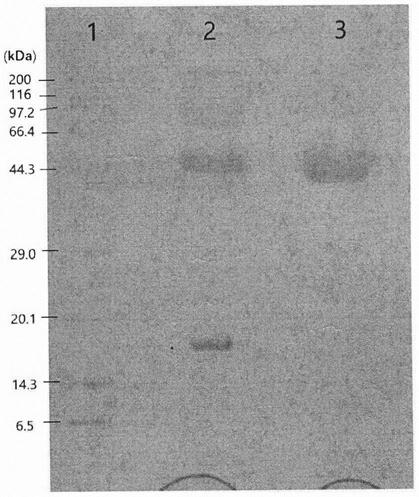
[0066] Equilibrate the molecular sieve column with 20 mM NaAc-HAc pH 4.0 buffer until the effluent pH is 4.0. The PEG reactant was sampled on the chromatography column, and the molecular sieve chromatography column was equilibrated with buffer 20mM NaAc-HAc pH4.0. The absorption peak of the target protein was collected according to the ultraviolet absorption value for protein quantification and electrophoresis detection.
[0067] 2) Heparin column chromatography
[006...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com