Zika virus E protein conjugate vaccine and preparation method thereof
A protein-binding vaccine, Zika virus technology, applied in the fields of botanical equipment and methods, biochemical equipment and methods, viruses, etc., can solve problems such as long-term toxicity, non-degradability, and limited clinical application of nanoparticle vaccines
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074] Embodiment 1: Expression and purification of E protein
[0075] The gene sequence of the E protein is derived from the envelope protein of the Zika virus BeH818995 strain (Genebank number: KU365777); 6 histidine residues are fused to the C-terminus of the E protein gene sequence source (1-409 amino acid residues). After the synthetic gene sequence was cloned into pET21a expression vector, it was transformed into Escherichia coli BL21(DE3) for high-level expression. Synthetic DNA sequences were determined by restriction enzyme digestion and DNA sequence analysis. Activate Escherichia coli cells expressing E protein, inoculate 200ml LB medium at a ratio of 1%, and culture with shaking until OD 600 Equal to 0.6-0.8, add IPTG with a final concentration of 0.5mmol / L, and induce at 37°C for 3-4 hours. Centrifuge at 4° C. and 10000 rpm for 10 minutes, collect the bacterial cells, and resuspend in 50 mM Tris-HCl buffer (pH 8.0). Ultrasonication was performed for 1 hour in an...
Embodiment 2
[0078] Embodiment 2: Preparation and purification of vaccine
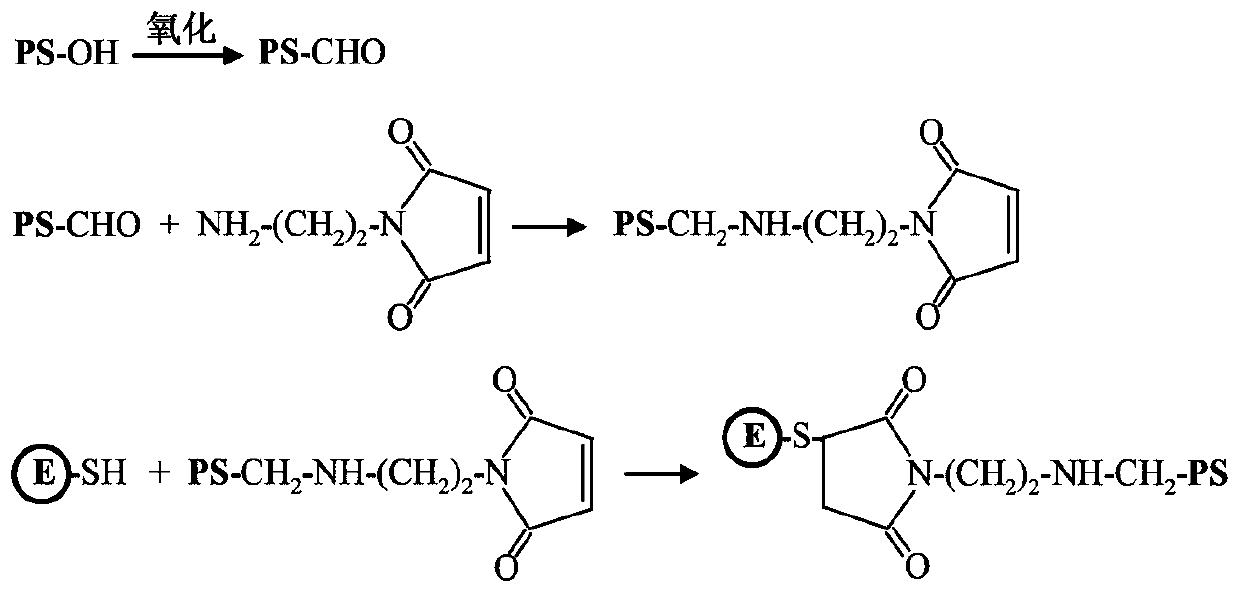
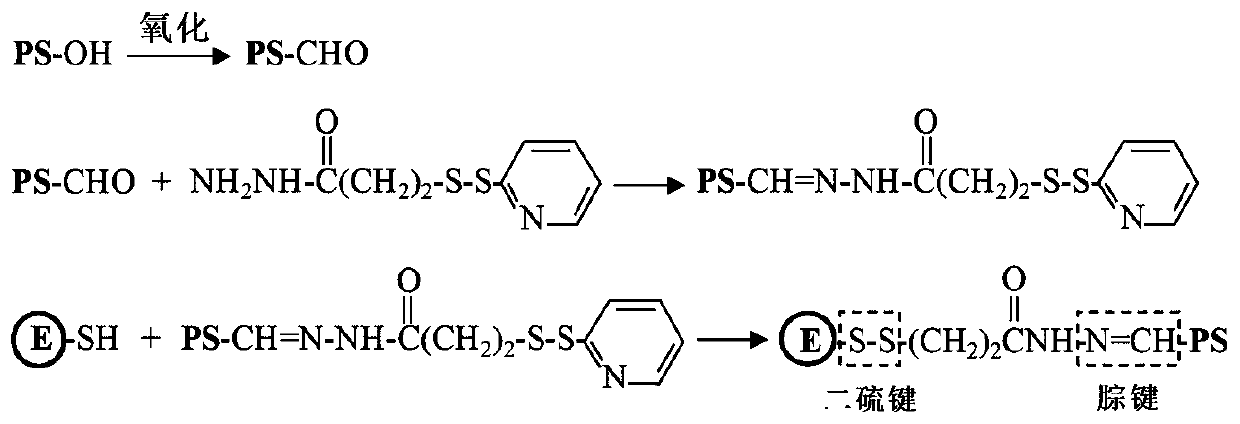
[0079] (1) Preparation of E-PS-1
[0080] E protein has a cysteine residue located on the surface of the E protein molecule; the sulfhydryl group of cysteine can be used to covalently bind β-glucan. Such as figure 2 Sodium periodate was dissolved in a volume of 5 mL of 50 mM acetate buffer (pH 5.6) to a final concentration of 40 mM. β-glucan (PS-OH) was dissolved in a volume of 5 ml of 50 mM acetate buffer (pH 5.6) to make a final concentration of 10 mg / mL. The two were mixed and placed at room temperature in the dark for 20 minutes. Subsequently, unreacted sodium periodate was removed by dialysis against PBS buffer (pH 7.4). Oxidized β-glucan (5 mL) with a concentration of 2 mg / mL and aminoethyl-maleimide ester (5 mL) with a concentration of 0.2 mg / mL were reacted overnight in PBS buffer (pH 7.4), and the reaction temperature is 4°C. Unreacted aminoethyl-maleimide ester was removed by dialysis against P...
Embodiment 3
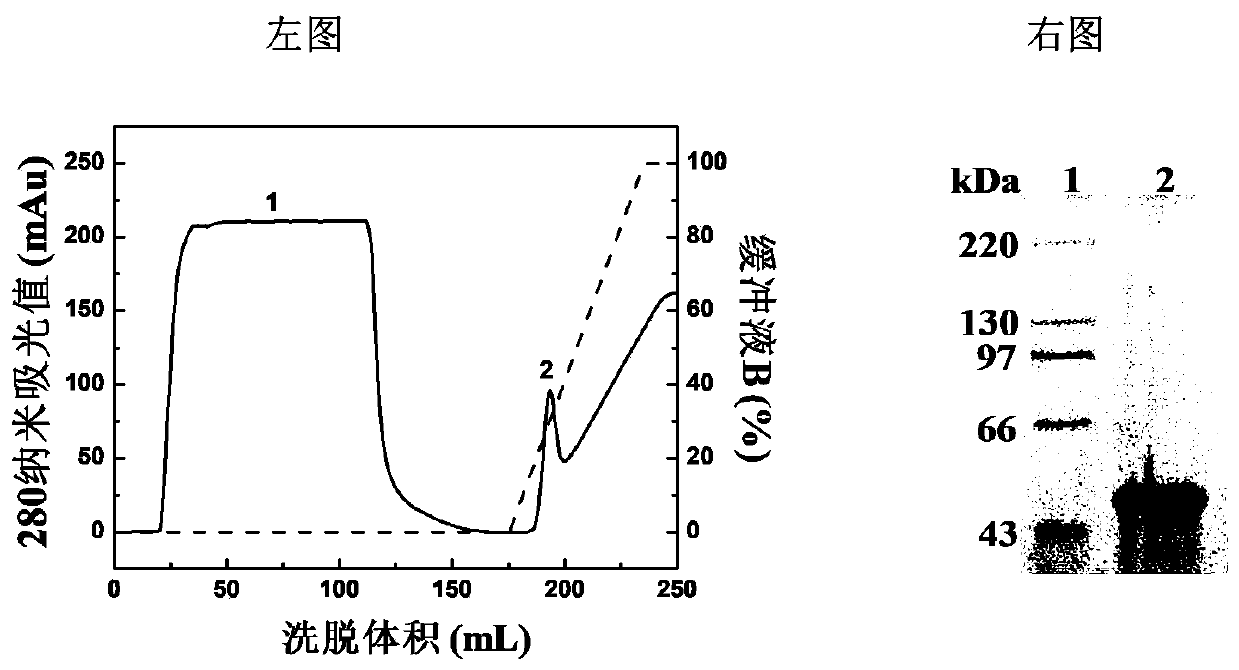
[0085] Embodiment 3: the purity of gel filtration analysis vaccine
[0086] E-PS-1 and E-PS-2 were identified by analytical Superdex 200 gel filtration column (1.0cm×30cm), the eluent was PBS buffer (pH 7.4), and the flow rate was 0.5mL / min. Such as Figure 5 As shown, E protein, E-PS-1 and E-PS-2 all present a single elution peak. Compared with the elution peak of E protein (15.4mL), the elution time of E-PS-1 and E-PS-2 was obviously earlier. This indicates that covalent attachment to β-glucan significantly enhances the hydration volume of E protein. The peak elution time of E-PS-1 and E-PS-2 was 7.7mL, indicating that the hydrazone bond and disulfide bond as connecting bridges did not change the hydration volume of the conjugate.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com