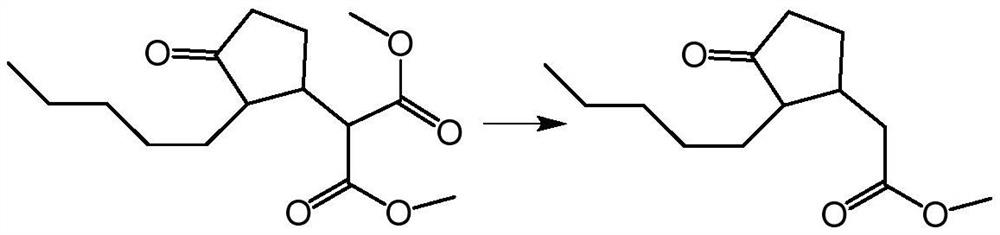
Preparation method of 3-(3-oxo-2-pentyl) cyclopentyl dimethyl malonate
A technology of dimethyl cyclopentyl malonate and dimethyl malonate, which is applied in the field of catalysis, can solve the problems of large amount of catalyst, corrosive equipment, and unfriendly environment, so as to reduce equipment investment and waste water , post-processing simple effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
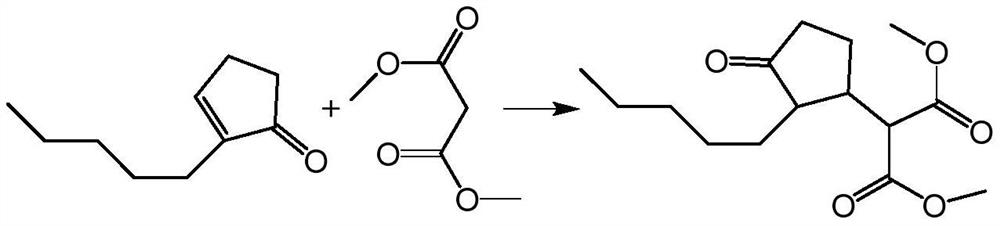
[0064] (1) Preparation of intermediate 3-(3-oxo-2-pentyl) dimethyl cyclopentylmalonate
[0065] Take by weighing 100g methyl alcohol, 2.63g rhodium chloride hydrate (0.01mol), 11.52g bis-diphenylphosphine methane (the mol ratio of rhodium chloride and bis-diphenylphosphine methane is 1:3) in there-necked flask, under nitrogen Under the atmosphere, the temperature was raised to 30°C and stirred for 60min to prepare a transition metal complex catalyst; the temperature of the transition metal complex catalyst solution was lowered to 0°C, and 152g (1mol) of 2-pentyl-2-cyclopentenone, acetone, and Dimethyl diacid 145.2g (1.1mol), L-proline 3.45g (the molar ratio of catalyst and L-proline is 1:3), keep warm for 3 hours, GC detects the content of reaction solution, 2-pentyl - If the total residual amount of 2-cyclopentenone is less than or equal to 0.5%, stop the reaction.
[0066] Rectification gives intermediate 3-(3-oxo-2-pentyl) dimethyl cyclopentylmalonate 268.68g, GC content 9...
Embodiment 2
[0070] (1) Preparation of intermediate 3-(3-oxo-2-pentyl) dimethyl cyclopentylmalonate
[0071] Take by weighing 100g dichloromethane, 2.07g ruthenium trichloride (0.01mol), 19.92g1,2-bisdiphenylphosphine ethane (the molar ratio of ruthenium trichloride and 1,2-bisdiphenylphosphine ethane 1:5) in a three-necked flask, heated to 20°C under nitrogen atmosphere and stirred for 80 minutes to prepare a transition metal complex catalyst; cool the transition metal complex catalyst solution to -2°C, and add 2-pentyl -2-cyclopentenone 152g (1mol), dimethyl malonate 198g (1.5mol), L-phenylalanine 8.26g (the molar ratio of catalyst and L-phenylalanine is 1:5), Incubate for 4 hours, detect the content of the reaction solution by GC, if the total residual amount of 2-pentyl-2-cyclopentenone is ≤0.5%, stop the reaction.
[0072] Rectification obtains intermediate 3-(3-oxo-2-pentyl) dimethyl cyclopentylmalonate 272.16g, GC content 98.69%, reaction process yield is 94.46% (according to 2-pen...
Embodiment 3
[0076] (1) Preparation of intermediate 3-(3-oxo-2-pentyl) dimethyl cyclopentyl malonate
[0077] Take by weighing 100g toluene, 7.85g copper acetylacetonate (0.03mol), 83.66g1,2-bisdiphenylphosphine ethane (the molar ratio of acetylacetonate copper and 1,2-bisdiphenylphosphine ethane is 1:7 ) in a three-necked flask, heated to 40°C and stirred for 80 minutes under nitrogen atmosphere to prepare a transition metal complex catalyst; cool the transition metal complex catalyst solution to -5°C, and add 2-pentyl-2-cyclo Pentenone 152g (1mol), dimethyl malonate 396g (3.0mol), L-4-hydroxyproline 31.47g (the molar ratio of catalyst and L-4-hydroxyproline is 1:8), Incubate for 5 hours, detect the content of the reaction solution by GC, if the total residual amount of 2-pentyl-2-cyclopentenone is ≤0.5%, stop the reaction.
[0078] The rectification obtained 266.42 g of the addition reaction intermediate, the GC content was 98.14%, and the reaction process yield was 91.95% (based on 2-p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com