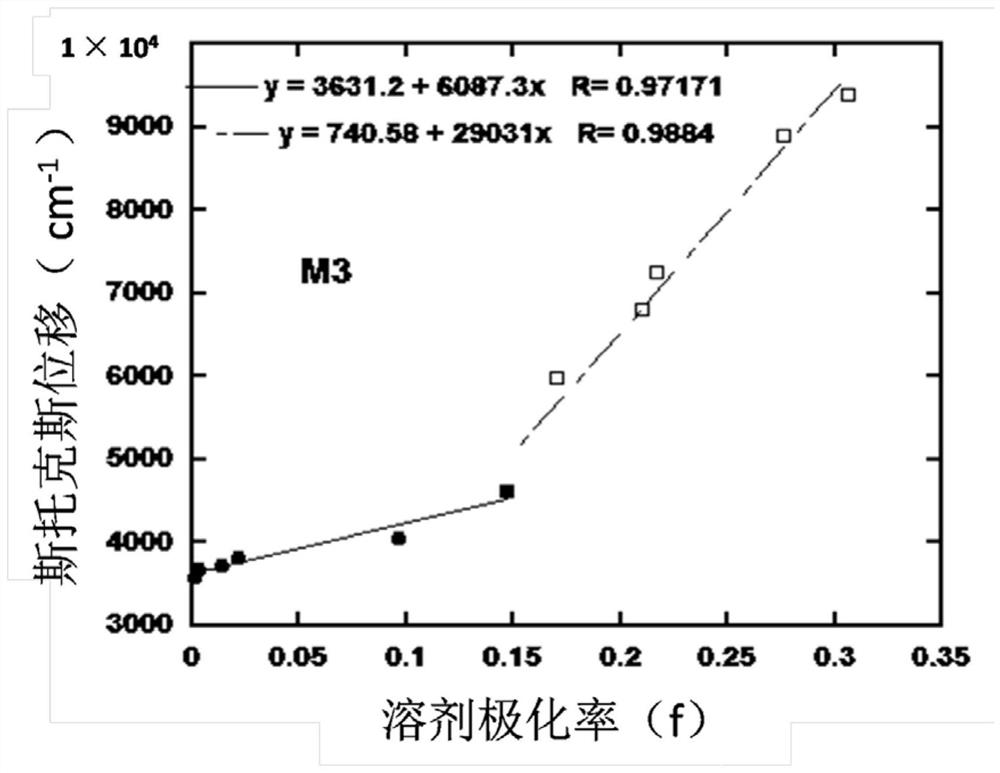
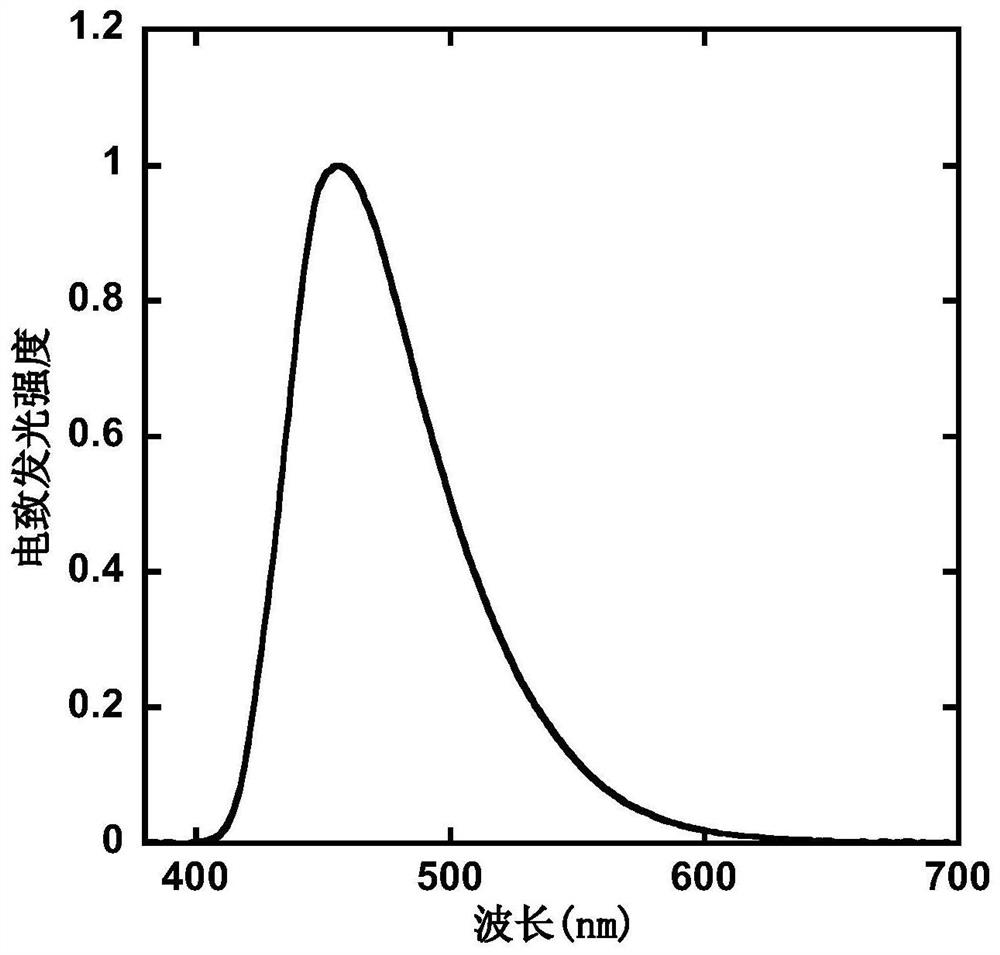
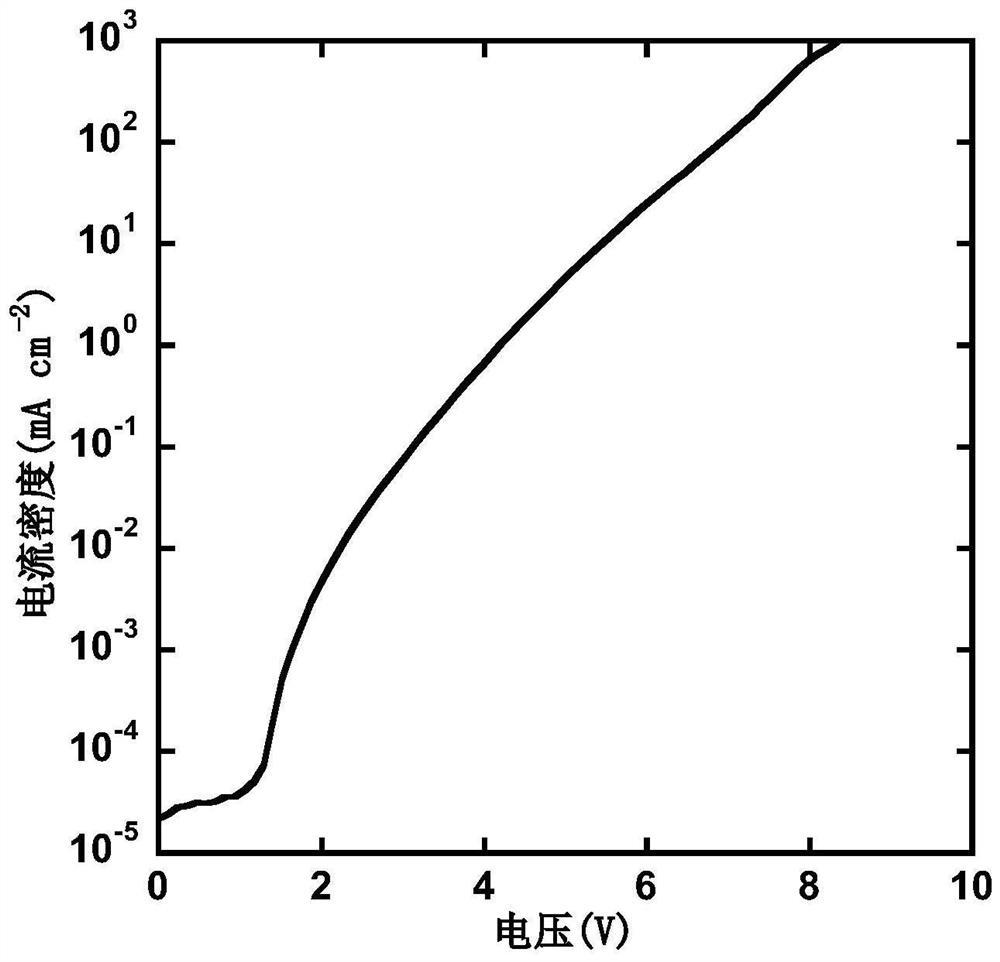
D-A type light-emitting small molecule containing acridine and phenanthroimidazole and application thereof in electroluminescent device
A technology of phenanthroimidazole and small molecules, applied in D-A type luminescent small molecules and its application in electroluminescent devices, can solve the problems of only 62.5% exciton utilization rate, unfavorable practical application, and efficiency roll-off, etc. Achieve high exciton utilization, high carrier mobility, and high fluorescence quantum yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Preparation of compound M1
[0027] The structural formula and synthetic route of compound M1 are shown in the figure below, and the specific synthetic method is as follows:
[0028]
[0029] (1) Synthesis of compound 1
[0030] Under the protection of nitrogen, 9,10-dihydro-9,9-diphenylacridine (10mmol), 9,10-dibromoanthracene (10mmol), sodium tert-butoxide (25mmol), catalyst tridibenzylidene Dipalladium acetone (Pd 2 (dba) 3 , 0.5mmol) and ligand tri-tert-butylphosphine (1mmol) were added to 50ml of toluene, stirring was started and heated to 120°C, and reacted for 10 hours. After the reaction, the product was extracted with ethyl acetate, washed three times with saturated sodium chloride solution, dried with anhydrous sodium sulfate, the dried solution was filtered, and the solvent was spin-dried by a rotary evaporator to obtain a crude product. The crude product was separated and purified by silica gel chromatography column, and the eluent was a mixed solvent...
Embodiment 2
[0038] Preparation of Compound M2
[0039] The structural formula and synthetic route of compound M2 are shown in the figure below, and the specific synthetic method is as follows:
[0040]
[0041] (1) Synthesis of Compound 4
[0042] Under the protection of nitrogen, 9,10-dihydro-9,9-dimethylacridine (10mmol), 1,4-dibromobenzene (10mmol), sodium tert-butoxide (25mmol), catalyst tridibenzylidene Dipalladium acetone (Pd 2 (dba) 3 , 0.5mmol) and ligand tri-tert-butylphosphine (1mmol) were added to 50ml of toluene, stirring was started and heated to 120°C, and reacted for 10 hours. After the reaction, the product was extracted with ethyl acetate, washed three times with saturated sodium chloride solution, dried with anhydrous sodium sulfate, the dried solution was filtered, and the solvent was spin-dried by a rotary evaporator to obtain a crude product. The crude product was separated and purified by silica gel chromatography column, and the eluent was a mixed solvent of ...
Embodiment 3
[0050] Preparation of compound M3
[0051] The structural formula and synthetic route of compound M3 are shown below, and the specific synthetic method is as follows:
[0052]
[0053] (1) Synthesis of Compound 7
[0054] Under the protection of nitrogen, 9,10-dihydro-9,9-dimethylacridine (10mmol), 1,3-dibromobenzene (10mmol), sodium tert-butoxide (25mmol), catalyst tridibenzylidene Dipalladium acetone (Pd 2 (dba) 3 , 0.5mmol) and ligand tri-tert-butylphosphine (1mmol) were added to 50ml of toluene, stirring was started and heated to 120°C, and reacted for 10 hours. After the reaction, the product was extracted with ethyl acetate, washed three times with saturated sodium chloride solution, dried with anhydrous sodium sulfate, the dried solution was filtered, and the solvent was spin-dried by a rotary evaporator to obtain a crude product. The crude product was separated and purified by silica gel chromatography column, and the eluent was a mixed solvent of petroleum ethe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com