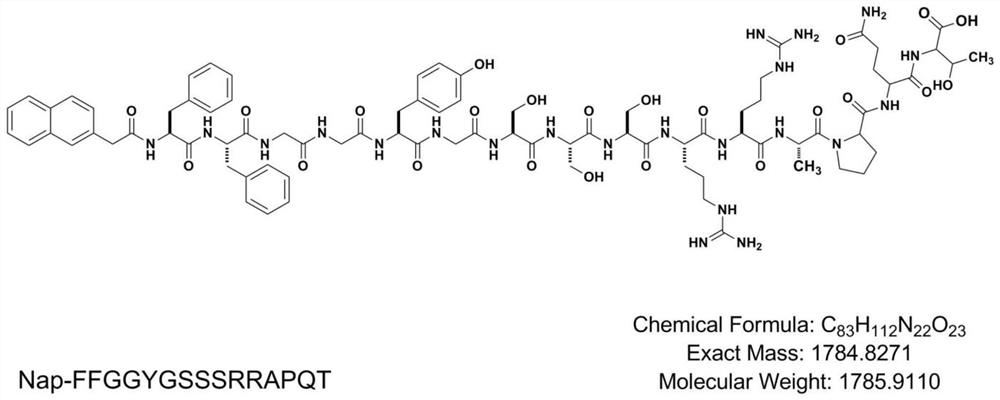
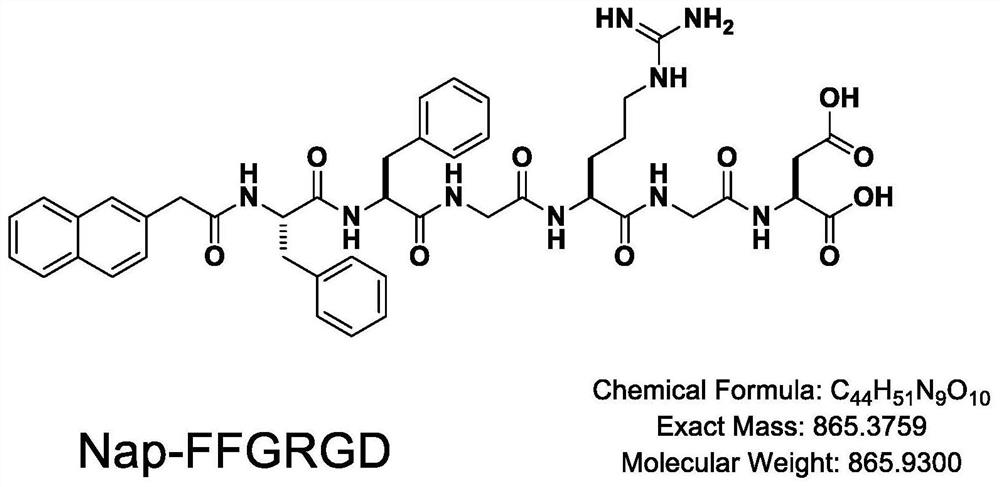
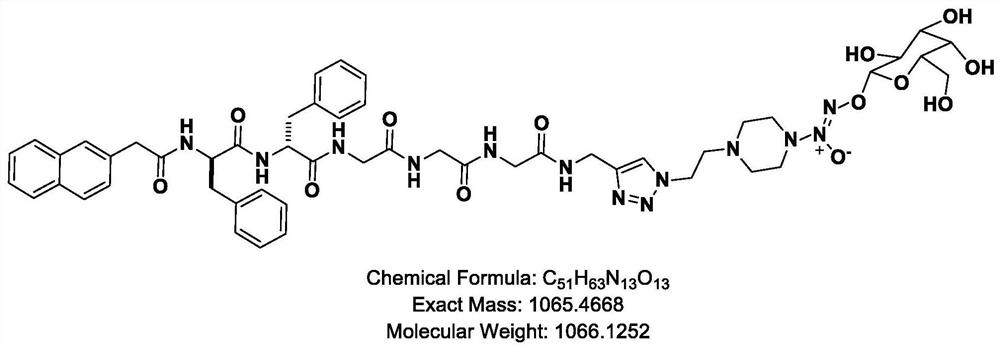
Vascular stent capable of promoting vascular cell proliferation and secreting extracellular matrix, preparation method of vascular stent and active artificial blood vessel
A vascular stent and vascular cell technology, applied in the field of active artificial blood vessels, can solve the problems of limited ability of passage growth and secretion of extracellular matrix, reduced cell growth and vitality, bacterial contamination, etc., to reduce the risk of infection and improve compatibility. Sex, improve activity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] A PCL vascular stent capable of promoting vascular cell proliferation and secreting extracellular matrix modified by IGF-1 polypeptide self-assembly, the preparation method comprising the following steps:
[0048] 1. Bracket processing:
[0049] (1) The inner layer of the stent was prepared by melt spinning, and the specific operation steps were as follows: Weigh 5.0 g of PCL with a molecular weight of 80,000, place it in a closed stainless steel syringe wrapped in a fuser, and heat it at 100°C for 1 hour; A stainless steel tube (receiving rod) with a diameter of 2mm is connected to the rotating motor, the distance between the syringe needle and the receiving rod is 5mm, the flow rate of the PCL melt is 0.5mL / h, the rotating speed of the receiving rod is 300r / min, and the translation speed of the receiver is 10mm / s, the spinning time is 5min, the prepared fiber angle is 50°, the fiber diameter is 60μm, and the inner layer fiber with wall thickness is 300μm;
[0050] (2...
Embodiment 2
[0074] A PCL / collagen composite vascular stent capable of promoting vascular cell proliferation and secreting extracellular matrix for slow release of IGF-1 polypeptide, the preparation method comprising the following steps:
[0075] First, the PCL / collagen composite stent was prepared by electrospinning technology. PCL was dissolved in a mixed solvent of chloroform and methanol (5:1 by volume) at a concentration of 10% (w / v). Type I collagen was dissolved in hexafluoroisopropanol at a concentration of 8% (w / v). IGF-1 polypeptide was dissolved in deionized water at a concentration of 2mg / mL. Add the IGF-1 polypeptide solution to the collagen solution, the volume ratio is 4:1, and mix well.
[0076] Fill the PCL solution and collagen / IGF-1 solution into two 10-mL syringes with 21-G needles, respectively. The two syringes are respectively installed on the two syringe pumps, and the rotating receiving rod is a stainless steel tube with a diameter of 2-6mm. The distance betwee...
Embodiment 3
[0083] A VEGF growth factor modified PCL / gelatin composite vascular stent capable of promoting vascular cell proliferation and secreting extracellular matrix, the preparation method comprising the following steps:
[0084] Firstly, the PCL / gelatin composite vascular stent was prepared by electrospinning technology. PCL was dissolved in a mixed solvent of chloroform and methanol (5:1 by volume) at a concentration of 25% (w / v). A gelatin solution was then prepared by dissolving gelatin in hexafluoroisopropanol at a concentration of 6% (w / v). Add the two solutions into two 10mL syringes, use 21G needles, and install them on two syringe pumps respectively. The high-voltage power supplies connected to the two syringes of PCL and gelatin are respectively, PCL11kV, and gelatin 17kV. The distances between the needles of the two syringes and the receiving rods are 25 cm for PCL and 15 cm for gelatin. The two materials were spun simultaneously to prepare the PCL / gelatin composite vasc...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fiber diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com