Synthesis method of heptafluoroisobutyronitrile
A technology of heptafluoroisobutyronitrile and synthesis method, which is applied in the field of synthesis of heptafluoroisobutyronitrile, can solve problems such as environmental pollution, increased production cost, and long route, and achieve simple and safe process operation, reaction conversion rate and yield High, the effect of high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
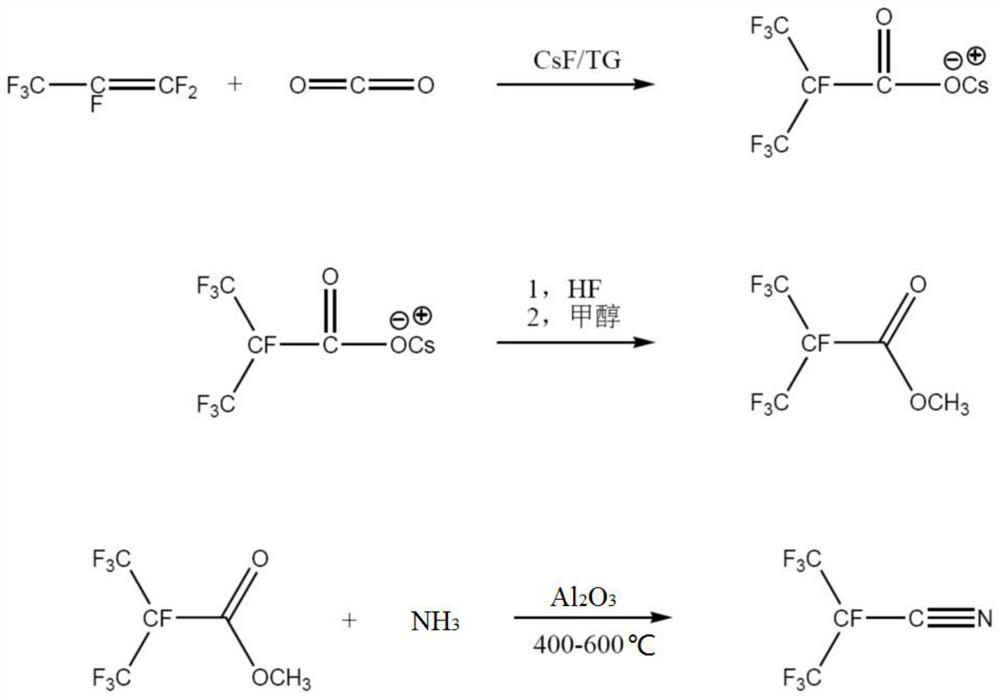
[0086] A kind of synthetic method of heptafluoroisobutyronitrile of the present invention, its synthetic route figure is as figure 1 shown. This synthetic method comprises the following steps:
[0087] Step (1): Synthesis of Heptafluoroisobutyrate
[0088] First, the reaction system is heated and vacuumized to remove water, and the whole system is purged with high-purity nitrogen to remove oxygen therein; the reactor is cooled to normal temperature, and under the protection of nitrogen, tetraethylene glycol dimethyl ether (222.28g, 1mol), dry cesium fluoride (89.6g, 0.59mol), stirred, heated to 70°C; then metered into hexafluoropropylene (HFP) (118.5g, 0.79mol) and carbon dioxide (44g, 1.00mol) , During the process, the pressure of the reactor is controlled at 0.1Mpa to 0.5Mpa, and the reaction is kept for 8 hours. After the reaction, separate the fluorocarbon layer (HFP dimer / trimer, about 21g), filter the TG layer (tetraethylene glycol dimethyl ether) to remove TG, wash t...
Embodiment 2
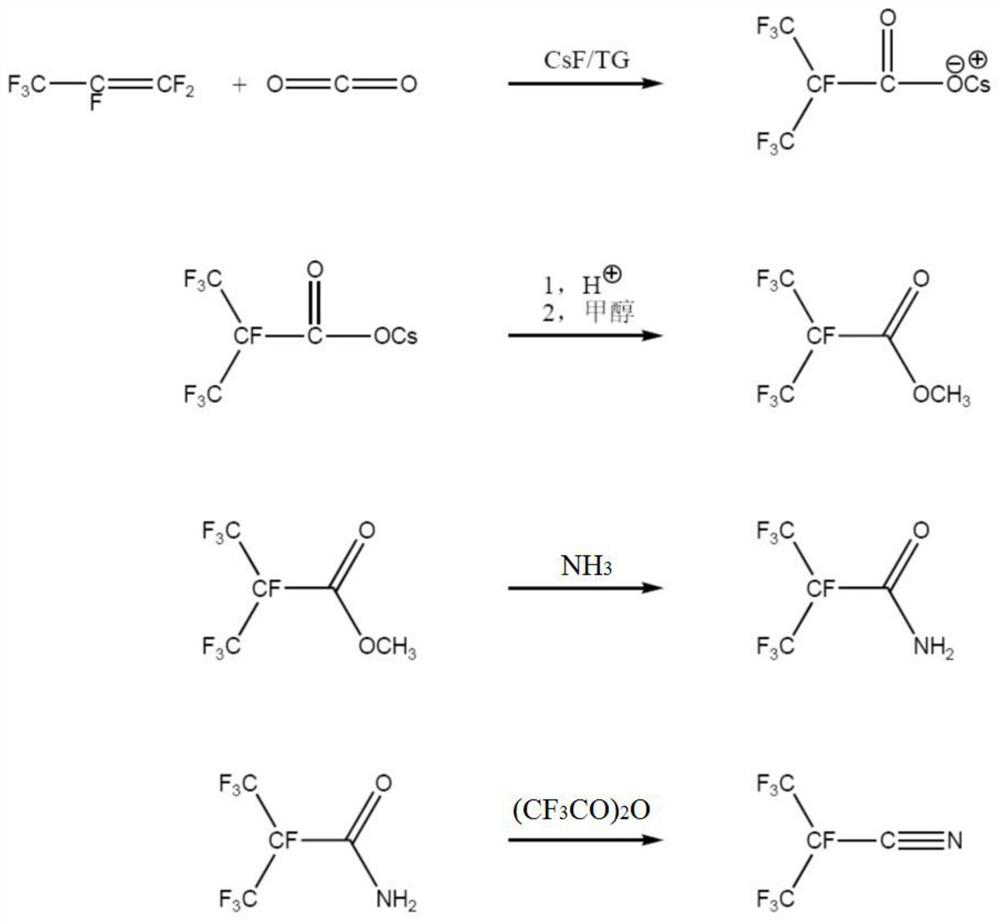
[0097] A kind of synthetic method of heptafluoroisobutyronitrile of the present invention, its synthetic route figure is as image 3 shown. This synthetic method comprises the following steps:
[0098] Step (1): Synthesis of Heptafluoroisobutyrate
[0099] First, the reaction system is heated and vacuumized to remove water, and the whole system is purged with high-purity nitrogen to remove oxygen therein; the reactor is cooled to normal temperature, and under nitrogen protection, tetraethylene glycol dimethyl ether (222.28g , 1mol), add dry cesium fluoride (90g, 0.59mol), stir, heat up to 70 ℃; ), the pressure of the reactor was controlled at 0.1MPa~0.2Mpa during the process, and the reaction was kept for 8 hours. After the reaction, separate the fluorocarbon layer (HFP dimer / trimer, about 21g), filter the TG layer (tetraethylene glycol dimethyl ether) to remove TG, wash the filter cake with benzene, and dry the filter cake to obtain heptafluoroisobutyl cesium base salt (1...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com