Mutant of epimerase and application of mutant
An epimerase and mutant technology, applied in the field of enzyme engineering, can solve the problems of poor temperature stability, unsuitable for industrial production, low fructose catalytic activity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1: Construction of mutants of D-psicose-3-epimerase
[0030] With the wild-type D-psicose-3-epimerase gene (GenBank: BAW27657.1) derived from Arthrobacter globiformis, the nucleic acid sequence (coding The amino acid sequence shown in SEQ ID NO: 2). The nucleic acid sequence was connected to the pET28a(+) expression vector to obtain the wild-type recombinant vector AgDAE-pET-28a. Using the wild-type recombinant vector as a template, V105I-F (SEQ ID NO: 5) and V105I-R (SEQ ID NO: 6) as primers, the KOD-Plus mutant kit was used to obtain the nucleus of mutant V105I by PCR site-directed mutation. nucleotide sequence. Concrete reaction steps and conditions are as follows:
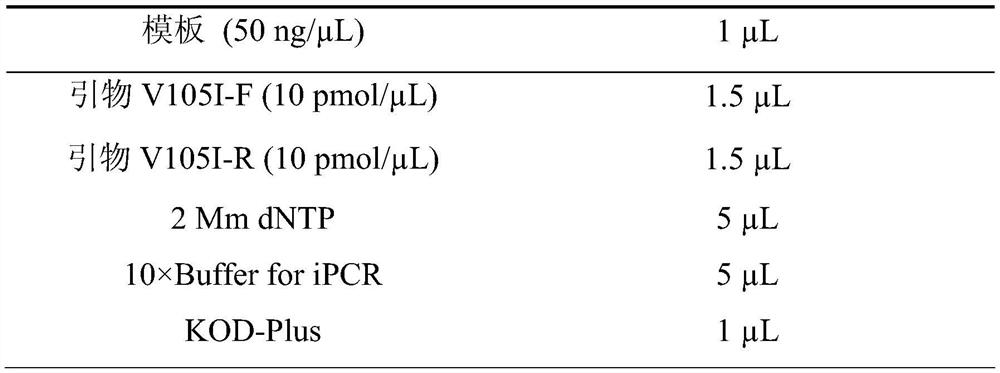
[0031] (1) PCR reaction, the reaction system and primer sequences are shown in Table 1 and Table 2 respectively. The underlined bases in Table 2 correspond to mutation points.
[0032] Table 1 PCR reaction system
[0033]
[0034]
[0035] Table 2 V105I-F and V105I-R primer sequences ...
Embodiment 2
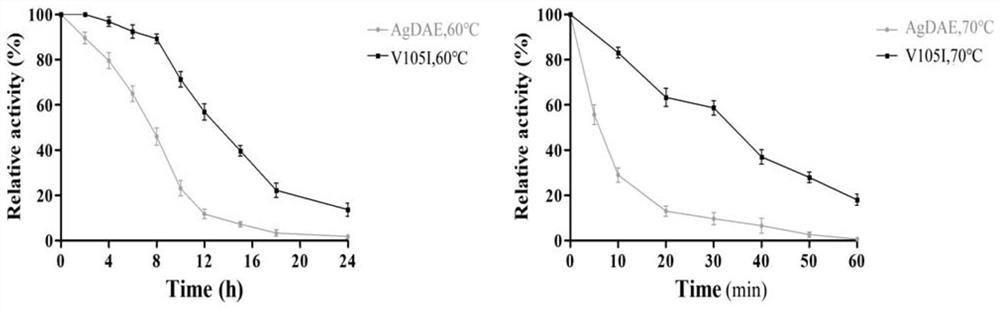
[0045] Example 2: Expression of D-psicose-3-epimerase mutants
[0046] (1) The recombinant vectors AgDAE-pET-28a and V105I-pET-28a were transformed into Escherichia coli BL21(DE3) to obtain BL21 / AgDAE recombinant bacteria and BL21 / V105I recombinant bacteria respectively.
[0047] (2) The above two kinds of recombinant bacteria were respectively inoculated into 5 mL LB seed medium (containing 50 μg / mL kanamycin), and cultured at 220 r / min at 37° C. for 12 hours. Inoculate 100mL of LB fermentation medium (containing 50μg / mL kanamycin) with 1% inoculum amount, prepare bacteria concentration OD600=0.6-0.8, add IPTG, final solubility is 0.5mM, at 16°C, 160r / min Under induction for 24h.
[0048] (3) Collect the cells by centrifugation at 5000r / min for 15min, resuspend the cells with Lysis Buffer (20mM Tris-HCl, 500mMNaCl, 20mM imidazole, 2mM DTT pH=7.4), add lysozyme (final solubility is 200μg / mL) , IPTG (final solubility: 1mM) placed on ice for 30min. The cell suspension was pla...
Embodiment 3
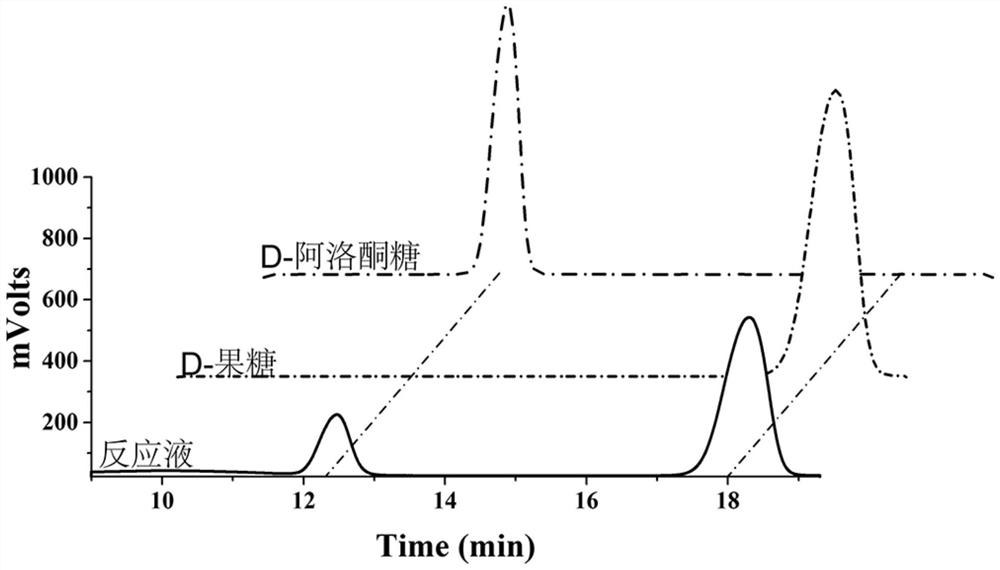
[0050] Embodiment 3: HPLC identification of D-psicose product
[0051] Add 20 mM Tirs-HCl (pH=7.4), 150 mM NaCl, 1% (w / v) fructose and an appropriate amount of enzyme to the 200 μL reaction system, and react at 60° C. for 10 min. After the reaction solution was boiled for 5 minutes, it was centrifuged to remove the precipitate, and the reaction solution was diluted to a suitable concentration into a liquid phase vial, and the product was quantitatively analyzed by HPLC. The determination conditions were:
[0052] Chromatograph: Agilent1260;
[0053] Detector: Evaporative Light Scattering Detector (Alltech Chrom, ELSD6000)
[0054] Injection: Agilent autosampler; injection volume 20 μL;
[0055] Chromatographic column: Prevail Carbohydrate ES column-W (5μm, 4.6×250mm, Agela Technologies, China); column temperature 40°C;
[0056] Mobile phase: 85% acetonitrile; flow rate 1 mL / min.
[0057] The result is as figure 1 As shown, the retention time of D-fructose is 18min, the re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com