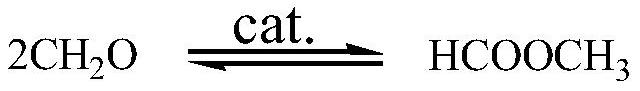Process for preparing paraformaldehyde with methanol as raw material
A paraformaldehyde and preparation process technology, applied in the direction of organic chemistry, can solve the problems of difficult recycling, heavy recycling system load, and many by-products, and achieve the effects of improving atomic utilization, increasing manufacturing costs, and reducing intermediate products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
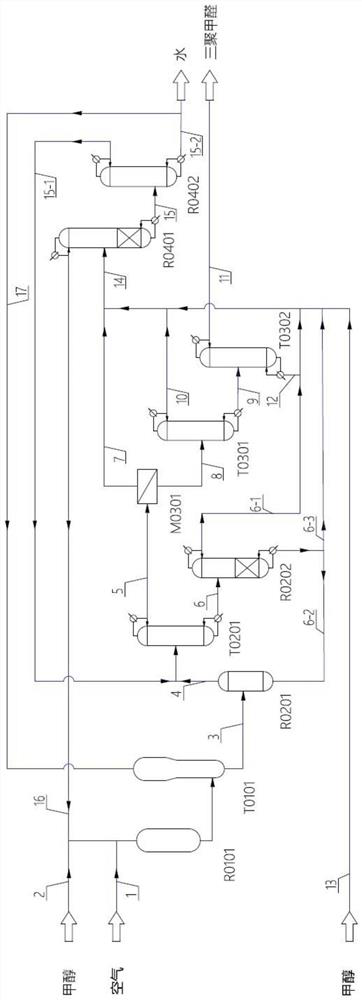
[0075] A process for preparing paraformaldehyde using methanol as a raw material, using methanol and methanol and methylal produced in the recovery section as raw materials to prepare concentrated formaldehyde with a concentration of more than 50%; the concentrated formaldehyde is synthesized under the catalysis of solid acid and acid, The formaldehyde aqueous solution containing paraformaldehyde is purified by rectification and membrane coupling process, and impurities such as methanol, methyl formate, formaldehyde, water, and formaldehyde polymers are separated to obtain pure paraformaldehyde.
[0076] The dilute aldehyde mixture and by-product impurities in the production process are converted into methylal through reactive distillation, and recycled to the reactor in the high-concentration formaldehyde production section, and part of the water generated in the recovery section is recycled to the formaldehyde absorption unit.
[0077] Described technique specifically compris...
Embodiment 2
[0103] The preparation process is performed by the following: formaldehyde reactor R0101 and formaldehyde absorption tower T0101; paraformaldehyde synthesis reactor R0201, paraformaldehyde concentration tower T0201 and dealcoholization reaction rectification tower R0202; membrane dehydration unit M0301, light removal tower T0301 And weight removal tower T0302; dilute aldehyde recovery reaction distillation tower R0401 and paraformaldehyde recovery distillation tower R0402.
[0104] In the formaldehyde reactor R0101, methanol and methylal mixture (methanol 2 and recycle stream 16) are evaporated, mixed with air (material 1) with a mass flow rate of 1825Kg / h, under the action of iron molybdenum oxidation catalyst, at 280 ℃, formaldehyde is generated under normal pressure, and the permeate stream 7 absorbs formaldehyde in the formaldehyde absorption tower T0101 to obtain a concentrated formaldehyde aqueous solution stream 3 with a formaldehyde concentration of 56.98%.
[0105] In...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com