A kind of haloamine polymer antibacterial and antiviral nanofiber membrane and preparation method thereof
A technology of nanofiber membranes and polymers, applied in synthetic fibers, conjugated synthetic polymer artificial filaments, fiber treatment, etc., can solve the problems of high reaction temperature, large solvent consumption, complex chlorination, etc., and achieve mild reaction conditions , Enhance adsorption, promote contact effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
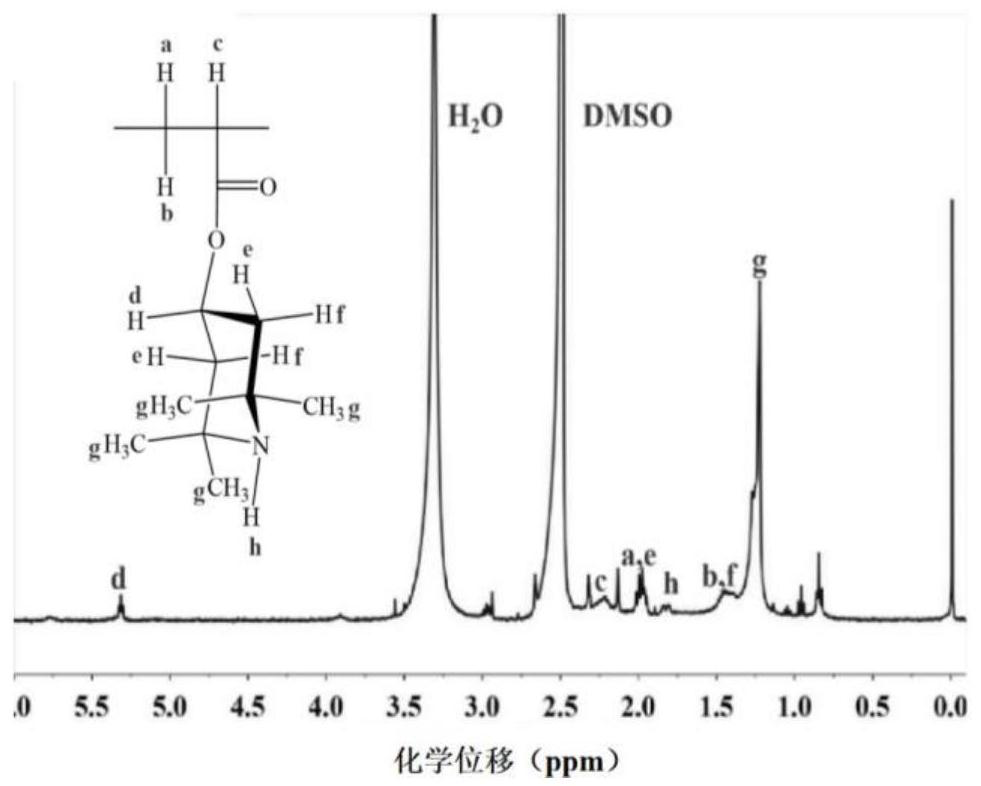
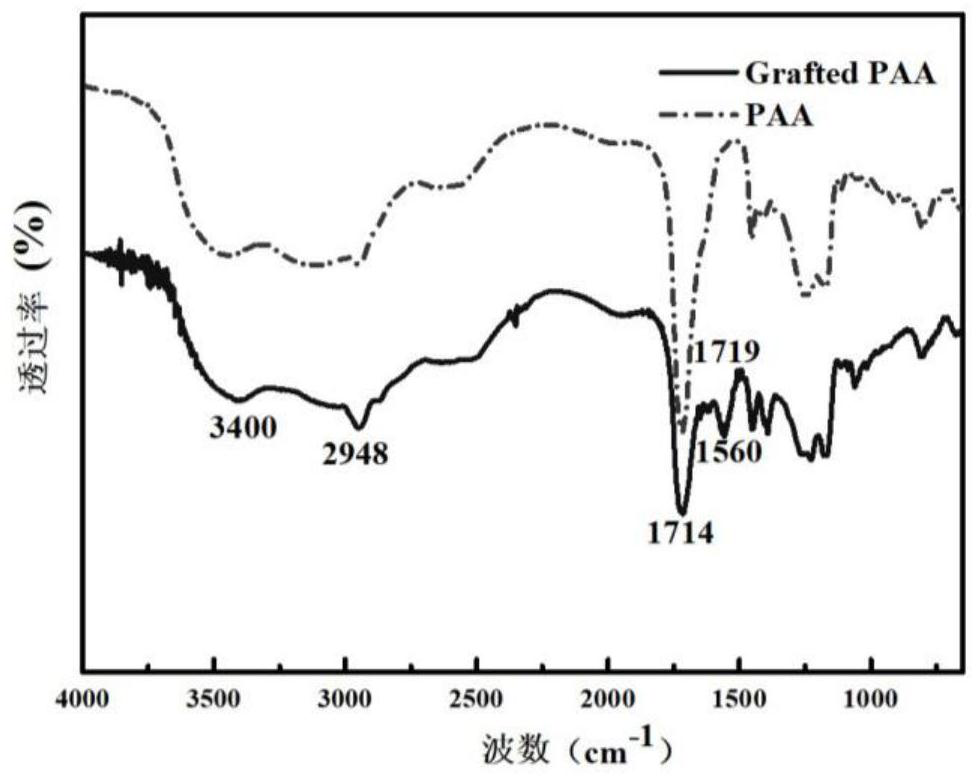
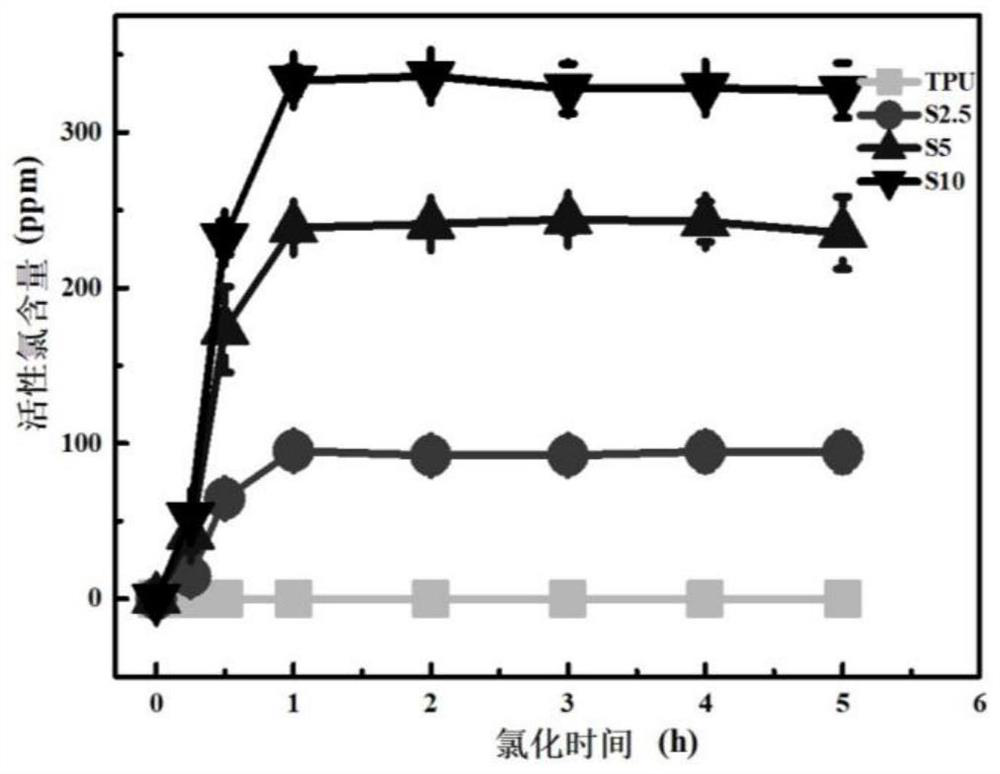
[0030] Dissolve 1.00 g polyacrylic acid in 30 mL N,N dimethylacetamide, stir at room temperature for 30 min, then add 0.10 g 1-ethyl-(3-dimethylaminopropyl) carbonyl Diimine hydrochloride and 0.01 g 4-dimethylaminopyridine were activated for 30 min; then 0.50 g tetramethylpiperidinol and 0.3 g N,N dimethylethanolamine were gradually dissolved in 5 mL N,N dimethyl Acetamide, dissolved liquid was added to the activated reaction system, and reacted at 40°C for 24 h. After the reaction, the solid was precipitated with methanol and the product was washed, then the product was dissolved in pure water for dialysis, and finally the dialysate was freeze-dried to obtain the product. Then mix 0.1 g of this product with 1 g of polyurethane in N,N dimethylacetamide solution to form a spinning solution with a total solute mass fraction of 20%, and obtain a nanofiber membrane by electrospinning, soak the nanofiber membrane Chlorination in 0.5wt% neutral sodium hypochlorite solution, then wa...
Embodiment 2
[0032] Dissolve 2.00 g of polyacrylic acid in 60 mL of N,N dimethylacetamide, stir at room temperature for 60 min, then add 0.30 g of dicyclohexylcarbodiimide and 0.03 g of 4-dimethylaminopyridine under ice-cooling conditions Activate for 1 h; then gradually dissolve 1.00 g tetramethylpiperidinol and 0.7 g triethylamine in 7 mL N,N dimethylacetamide, add the dissolved liquid into the activated reaction system, and react at room temperature for 48 h . After the reaction was completed, the solution was obtained by filtration, the solid was precipitated with acetone, and then the solid was dissolved with pure water. This was repeated three times, and finally the product was obtained by vacuum drying. Then 0.1 g of this product and 2 g of polyurethane were mixed in N,N dimethylacetamide solution to form a spinning solution with a total solute mass fraction of 17.5%, and the nanofiber membrane was obtained by electrospinning, and the nanofiber membrane was soaked in Chlorination i...
Embodiment 3
[0034] Dissolve 3.00 g of polyacrylic acid in 80 mL of N,N dimethylformamide, stir at room temperature for 100 min, then add 0.40 g of 1-ethyl-(3-dimethylaminopropyl)carbonyl di imine hydrochloride and 0.04 g 1,8-diazabicycloundec-7-ene were activated for 1.5 h; then 1.5 g tetramethylpiperidinol and 1 g diethylenetriamine were gradually dissolved in 8 mL N,N dimethylacetamide, the dissolved liquid was added to the activated reaction system, and reacted at room temperature for 48 h. After the reaction, the solid was precipitated with ethanol and washed, then the product was dialyzed, and finally the dialysate was freeze-dried to obtain the product. Then 0.1 g of the freeze-dried product was mixed with 2.5 g of polyurethane in N,N dimethylacetamide solution to form a spinning solution with a total solute mass fraction of 10%, and the nanofiber membrane was obtained by electrospinning. Soak in 1.5wt% neutral sodium hypochlorite solution for chlorination, then wash with water to ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com