Preparation method of carbon-coated lithium iron manganese phosphate positive electrode material
A carbon-coated technology of lithium iron manganese phosphate and cathode material, applied in the field of lithium ion batteries, can solve the problems of uneven product phase, wide particle size distribution, poor product consistency, etc. Control and reduce the effect of impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
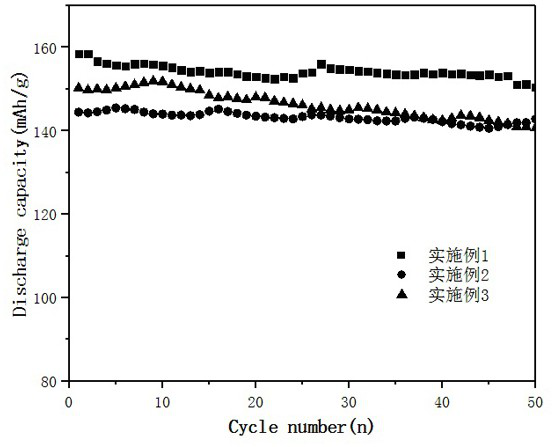
Embodiment 1
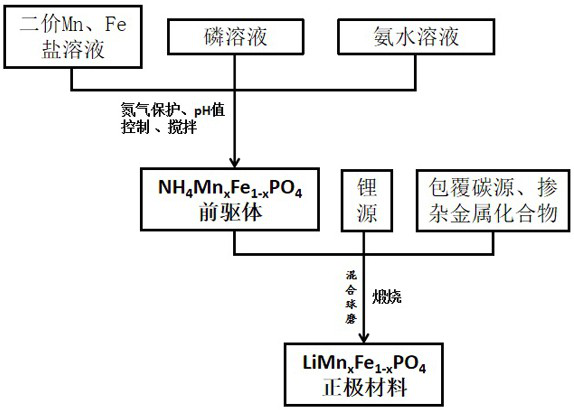
[0035] A method for preparing a carbon-coated lithium manganese iron phosphate cathode material, comprising the following steps:
[0036] (1) With deionized water as solvent, (CH 3 COO) 2 Mn and FeSO 4 According to the chemical formula LiMn 0.4 Fe 0.6 PO 4 The transition metal ion salt solution A is configured in the atomic ratio of Mn and Fe, wherein the metal ion concentration is 1.0 mol / L.
[0037] (2) With deionized water as solvent, configure NH 4 h 2 PO 4Solution B, wherein the phosphate concentration is 1.0mol / L.
[0038] (3) Ammonia solution C is configured to adjust the pH value of the system.
[0039] (4) Deionized water is used as the reaction bottom liquid, and ascorbic acid is added according to the stoichiometric ratio to inhibit Fe 2+ and Mn 2+ Oxidation, through nitrogen protection for more than 1 hour.
[0040] (5) The solution A and the solution B configured above are added dropwise in the reaction kettle at the same time. During the dropping proc...
Embodiment 2
[0046] (1) With deionized water as solvent, MnSO 4 and (CH 3 COO) 2 Fe according to the chemical formula LiMn 0.8 Fe 0.2 PO 4 The transition metal ion salt solution A is configured in the atomic ratio of Mn and Fe, wherein the metal ion concentration is 2.0 mol / L.
[0047] (2) With deionized water as solvent, configure (NH 4 ) 2 HPO 4 Solution B, wherein the phosphate concentration is 2.0mol / L.
[0048] (3) Ammonia solution C is configured to adjust the pH value of the system.
[0049] (4) Deionized water is used as the reaction bottom liquid, and glucose is added according to the stoichiometric ratio to inhibit Fe 2+ and Mn 2+ Oxidation, through nitrogen protection for more than 1 hour.
[0050] (5) The solution A and the solution B configured above are added dropwise in the reaction kettle at the same time. During the dropping process, the stirring rate in the reaction kettle is kept at 200rpm, and the solution C is added dropwise at the same time to keep the pH v...
Embodiment 3
[0056] (1) With deionized water as solvent, MnCl 2 and Fe(NO 3 ) 2 According to the chemical formula LiMn 0.3 Fe 0.7 PO 4 The transition metal ion salt solution A is configured in the atomic ratio of Mn and Fe, wherein the metal ion concentration is 3.0 mol / L.
[0057] (2) With deionized water as solvent, configure H 3 PO 4 Solution B, wherein the phosphate concentration is 3.0mol / L.
[0058] (3) Ammonia solution C is configured to adjust the pH value of the system.
[0059] (4) Deionized water is used as the reaction bottom liquid, and oxalic acid is added according to the stoichiometric ratio to inhibit Fe 2+ and Mn 2+ Oxidation, through nitrogen protection for more than 1 hour.
[0060] (5) The solution A and the solution B configured above are added dropwise in the reactor at the same time, during the dropping process, the stirring rate in the reactor is kept at 400rpm, and the solution C is added dropwise at the same time to keep the pH value of the system at 7,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com