Lysosome-targeted infrared two-window emission fluorescent dye as well as preparation method and application thereof
A fluorescent dye and lysosome technology, applied in the field of bioluminescent detection materials, can solve the problems of long-term toxicity and excretion time, and achieve the effect of rich raw materials, good photostability, and mild synthesis conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
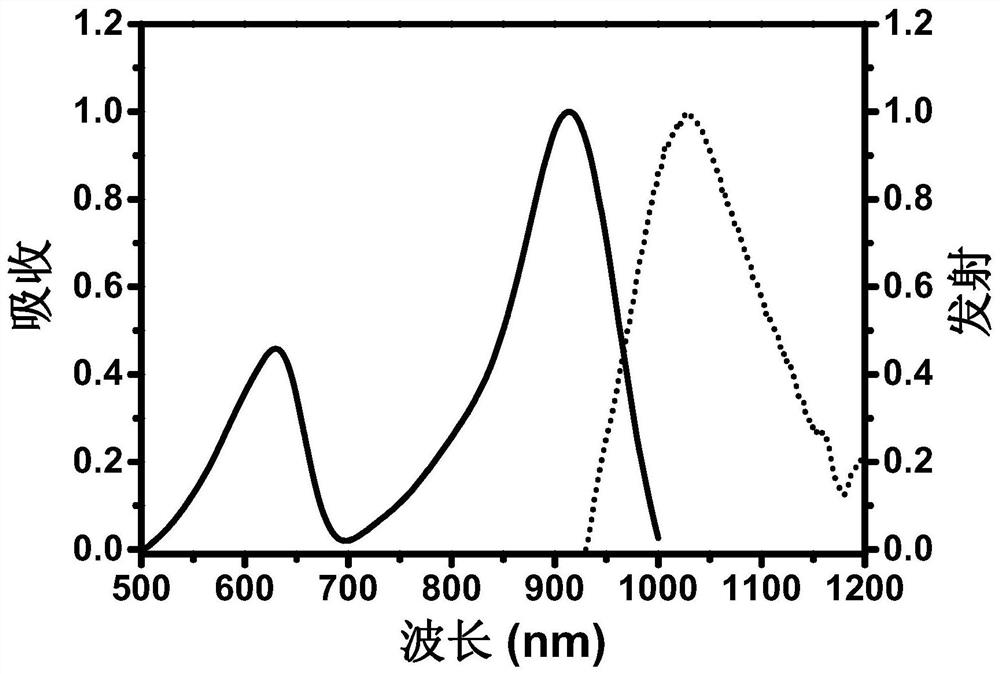
[0052]This example provides a method for preparing a lysosome-targeted infrared two-window emitting fluorescent dye, and the synthetic route is as follows:
[0053]
[0054] Specific steps include:
[0055] Synthesis of compound 2-1:
[0056] In 1.46g of 1-1 (about 10mmol), 1.91g (about 10mmol) of p-ethylaminoethanone and 30mL of pure ethanol mixed solution, slowly add 10mL of sodium hydride aqueous solution (containing 2.00g of sodium hydroxide). After reacting at room temperature for 12 hours, filter with suction. A yellow solid was obtained, which was washed with cold ethanol and dried in vacuo. 2.59 g of compound 2-1 was obtained (about 81% yield).
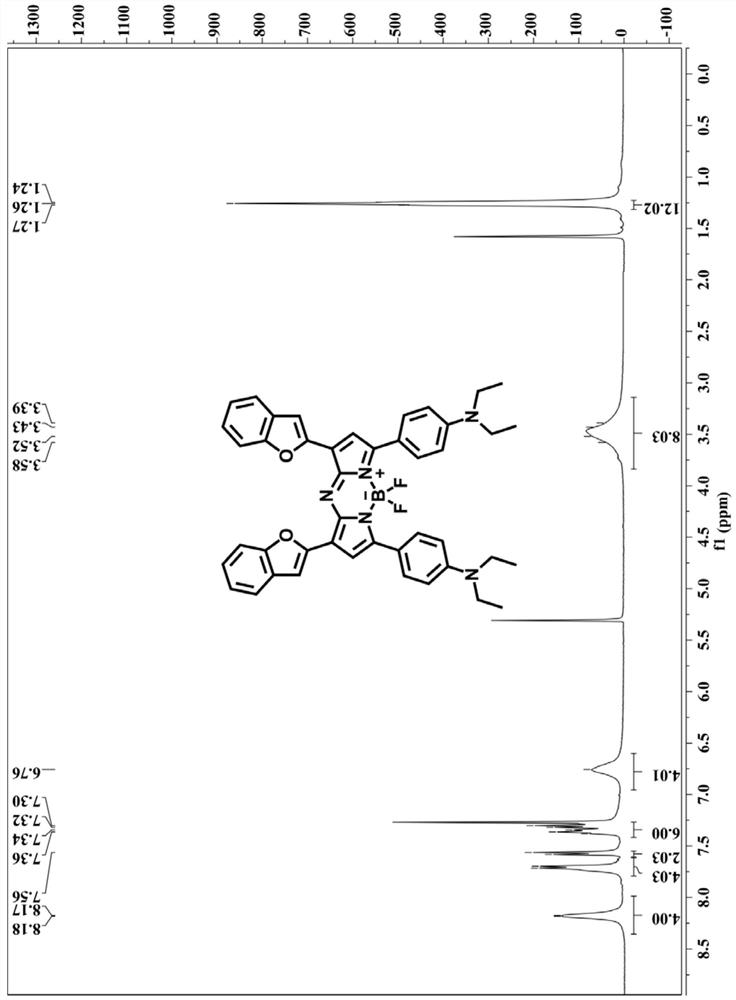
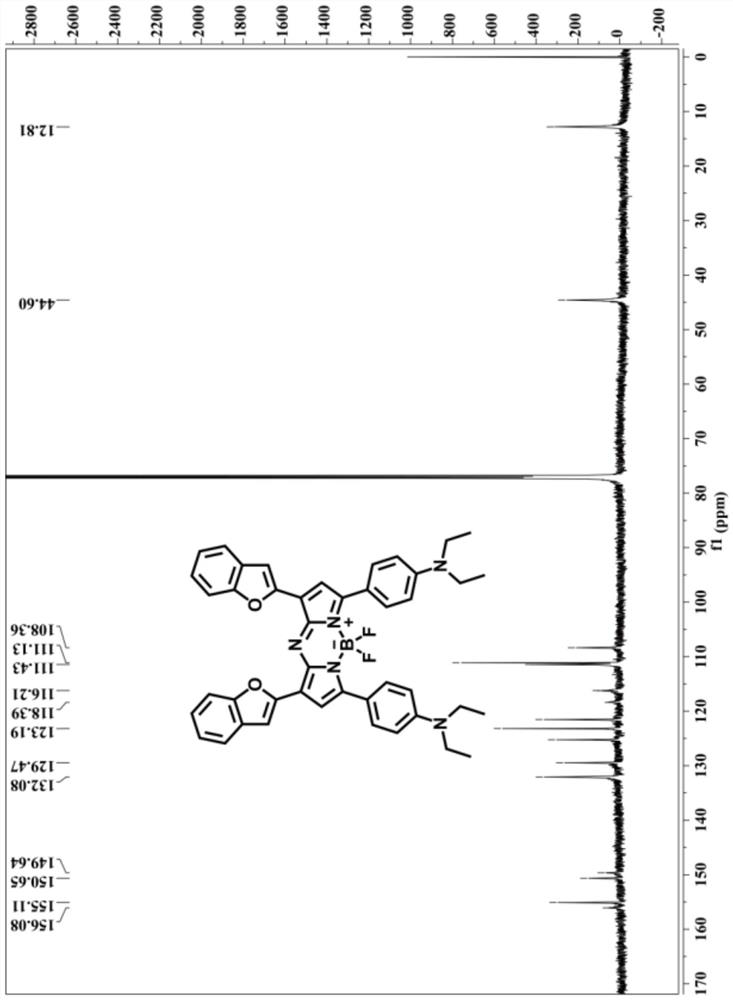
[0057] NMR of compound 2-1: 1 H NMR (400MHz, CDCl 3 )δ (ppm) = 8.04 (d, J = 6.4Hz, 2H), 7.77 (dd, J = 15.2, 2.4Hz, 1H), 7.66 (dd, J = 15.6, 3.2Hz, 1H), 7.59 (d, J=7.6Hz, 1H), 7.51(d, J=8.0Hz, 1H), 7.38–7.33(m, 1H), 7.26–7.22(m, 1H), 6.96(d, J=1.2Hz, 1H), 6.68 (d, J=7.2Hz, 2H), 3.44 (q, J=6.4Hz, 4H), 1.22 (t, J=5.2Hz,...
Embodiment 2
[0069] This example provides a method for preparing a lysosome-targeted infrared two-window emitting fluorescent dye, and the synthetic route is as follows:
[0070]
[0071] Specific steps include:
[0072] Synthesis of compound 2-2:
[0073] In the mixed solution of 1.62g 1-2 (about 10mmol), 1.91g (about 10mmol) of p-ethylaminoethanone and 30mL of pure ethanol, slowly add 10mL of sodium hydride aqueous solution (containing 2.00g of sodium hydroxide). After reacting at room temperature for 12 hours, filter with suction. A yellow solid was obtained, which was washed with cold ethanol and dried in vacuo. 3.02 g of compound 2-2 was obtained (about 90% yield).
[0074] Synthesis of Compound 3-2:
[0075] The mixed solution of 3.35g 2-2 (about 10mmol), 15mL nitromethane, 8mL diethylamine and 20mL pure ethanol was reacted overnight under reflux conditions (75°C), separated by silica gel column chromatography (developing agent ethyl acetate : Petroleum ether=4:1, v / v) 3.29g ...
Embodiment 3
[0081] This example provides a method for preparing a lysosome-targeted infrared two-window emitting fluorescent dye, and the synthetic route is as follows:
[0082]
[0083] Specific steps include:
[0084] Synthesis of compound 2-3:
[0085] In the mixed solution of 1.73g 1-3 (about 10mmol), 1.91g (about 10mmol) of p-ethylaminoethanone and 30mL of pure ethanol, slowly add 10mL of sodium hydride aqueous solution (containing 2.00g of sodium hydroxide). After reacting at room temperature for 24 hours, filter with suction. A yellow solid was obtained, which was washed with cold ethanol and dried in vacuo. 3.08 g of compound 2-3 was obtained (about 91% yield).
[0086] Synthesis of compound 3-3:
[0087] The mixed solution of 3.46g 2-3 (about 10mmol), 15mL nitromethane, 8mL diethylamine and 20mL pure ethanol was reacted overnight under reflux condition (70 ℃), separated by silica gel column chromatography (developing agent ethyl acetate : Petroleum ether=4:1, v / v) 3.30 g ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com