Submucosal injection solution composition for endoscope and preparation method
A composition and solution technology, which is applied in drug delivery, medical formulation, surgery, etc., can solve the problems of poor biocompatibility, high reagent concentration, and difficulty in injection, so as to reduce postoperative bleeding, excellent effect, and easy to use. The effect of the injection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] (1) Add 1000ml of water for injection, 0.864g of sodium dihydrogen phosphate, 0.475g of disodium hydrogen phosphate, 9.0g of sodium chloride, and 0.05g of indigo in the preparation container. After the solution is completely dissolved, use the sterilized 0.45μm solution. The filter element is filtered under high pressure, and then placed in a high-pressure steam sterilizer, sterilized at 121°C for 30 minutes, to obtain phosphate buffer;
[0038] (2) After the above solution is cooled, add 2.8 g of hyaluronic acid and 3.2 g of carboxymethyl chitosan into a phosphate buffer solution in a mixing tank, seal and stir until the solution is completely dissolved;
[0039] (3) Filling is carried out under aseptic conditions to obtain submucosal injection solution composition products for endoscopes.
Embodiment 2
[0041] (1) Add 1000 ml of water for injection, 0.578 g of sodium dihydrogen phosphate, 0.215 g of disodium hydrogen phosphate, 3.2 g of sodium chloride, and 0.005 g of trypan blue to the preparation container. After the solution is completely dissolved, use the sterilized solution The 0.45μm filter element is filtered under high pressure, and then placed in a high-pressure steam sterilizer, sterilized at 121°C for 30 minutes to obtain phosphate buffer;
[0042] (2) After the above solution is cooled, add 4.0 g of hyaluronic acid and 1.8 g of carboxymethyl chitosan into a phosphate buffer solution in a mixing tank, seal and stir until the solution is completely dissolved;
[0043] (3) Filling is carried out under aseptic conditions to obtain submucosal injection solution composition products for endoscopes.
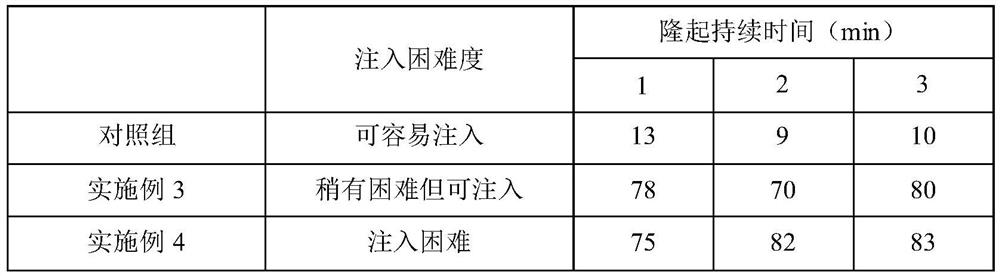
Embodiment 3
[0045] (1) Add 1000 ml of water for injection, 0.618 g of sodium dihydrogen phosphate, 0.215 g of disodium hydrogen phosphate, 7.50 g of sodium chloride, and 0.01 g of methylene blue into the preparation container. After the solution is completely dissolved, use a sterilized 0.45 μm solution. The filter element is filtered under high pressure, and then placed in a high-pressure steam sterilizer, sterilized at 121°C for 30 minutes to obtain phosphate buffer;
[0046] (2) After the above solution is cooled, add 1.3 g of hyaluronic acid and 2.7 g of carboxymethyl chitosan into a phosphate buffer solution in a mixing tank under a 100% clean environment, and seal and stir until the solution is completely dissolved;
[0047] (3) Filling is carried out under aseptic conditions to obtain submucosal injection solution composition products for endoscopes.
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of substitution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com