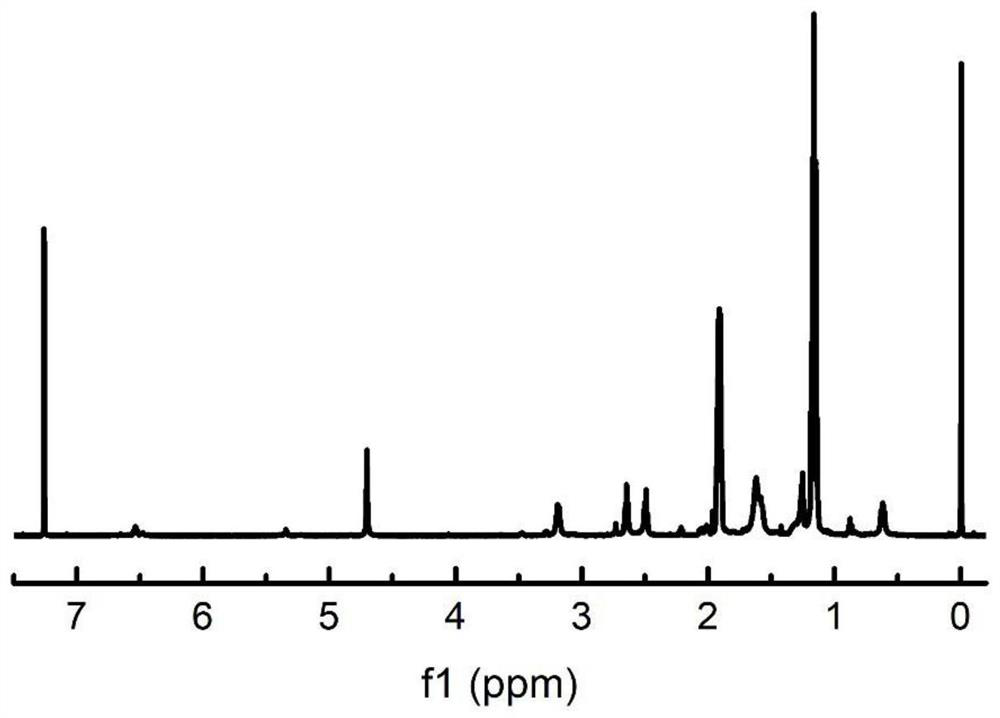
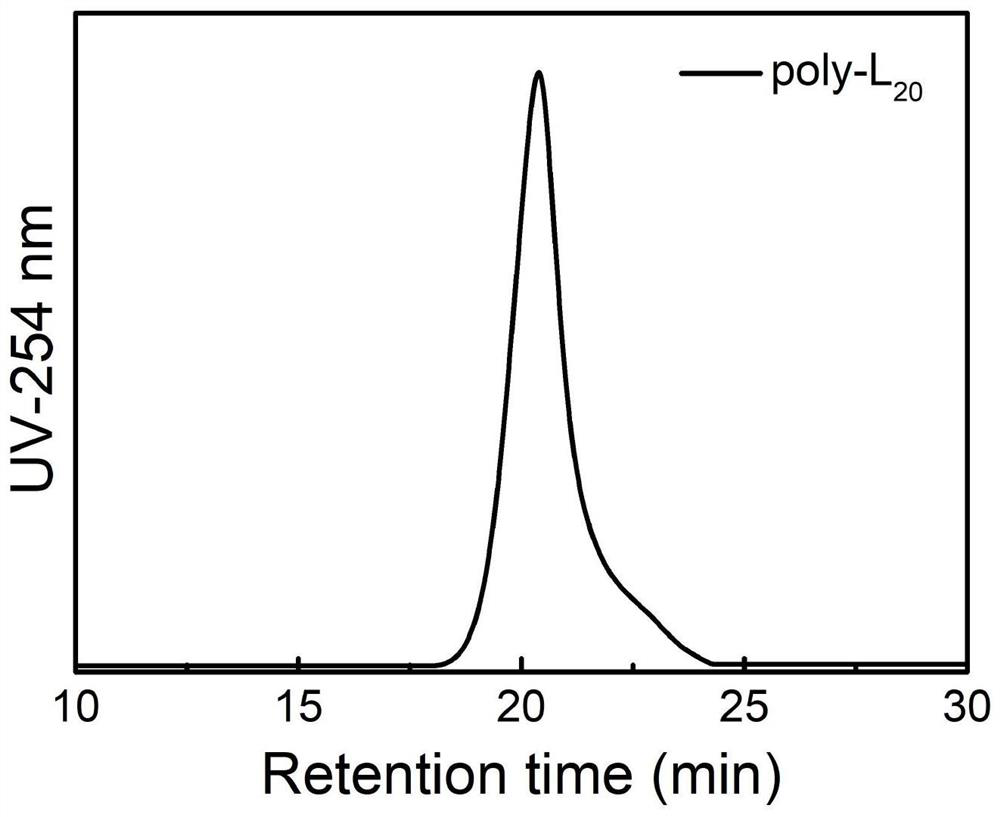
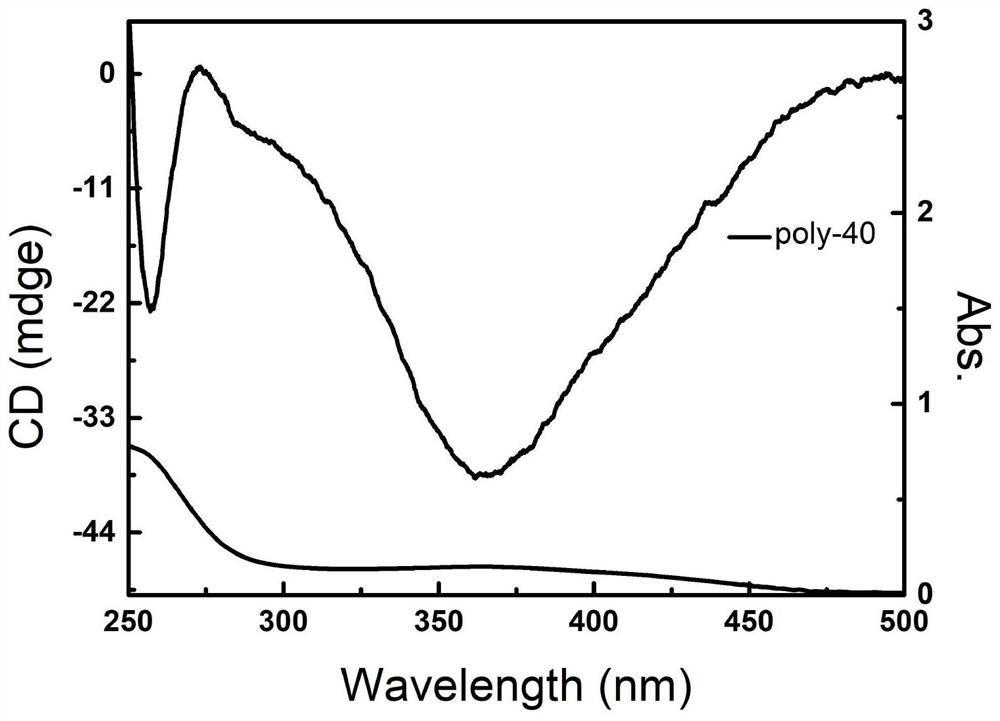
Cage type polysilsesquioxane-based star-shaped hybrid spiral polyisocyanide as well as preparation method and application of polysilsesquioxane-based star-shaped hybrid spiral polyisocyanide
A polyisonitrile and hybrid technology is applied in the field of cage-type polysilsesquioxane-based star-shaped hybrid helical polyisonitrile and its preparation, which can solve the problems of rare hybrid star-shaped polyisonitrile research and the like, and achieves The experimental conditions are not demanding, the preparation is simple, and the operation is convenient.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Embodiment 1: the preparation method of organic / inorganic hybrid palladium catalyst
[0042] a. Dissolve 0.17mmol (0.20g) of octaamino POSS and 4.09mmol (0.63g) of monopropynyl succinate into 50mL of dichloromethane, and add 2.73mmol (1.03g) of O-benzotriazepam Azole-tetramethyluronium hexafluorophosphate, 2.73 mmol (0.37 g) of 1-hydroxybenzotriazole and 10 mL of triethylamine. Under nitrogen atmosphere, react at room temperature for 24h, stop the reaction. The organic phase was taken with 30 mL of dichloromethane, washed three times with saturated ammonium chloride solution, saturated sodium bicarbonate solution, and saturated sodium chloride solution successively, dried by adding anhydrous sodium sulfate, and evaporated to remove the solvent to obtain a crude product. Add 2 mL of dichloromethane and precipitate with 20 mL of ether to obtain intermediate I; the structural formula is as follows:
[0043] In the formula
[0044] b. Add 2mmol (726.64mg) of bis(trie...
Embodiment 2
[0049] Example 2: Initiating Chiral C 10 Isonitrile Polymerization
[0050] Chiral C 10Isonitrile polymerization is carried out under anhydrous and oxygen-free conditions, and 1.60 mg (0.0003 mmol) of the palladium catalyst prepared in Example 1 is added in a 10 mL polymerization bottle, and the chiral C 10 Isonitrile isonitrile monomer 19.31mg (0.0614mmol), vacuumize and inflate with nitrogen for 3 times, add 1mL dry tetrahydrofuran, reflux reaction at 55°C for 8h, add 10mL methanol to quench, precipitate the polymer, wash with methanol Centrifuge 5 times to obtain a yellow flocculent precipitate, and vacuum-dry until the mass remains unchanged.
[0051] The structural formula of the phenylisonitrile monomer in the present embodiment is:
[0052]
[0053] The structural formula of the product obtained is:
[0054]
Embodiment 3
[0055] Example 3: Chiral C 10 Isonitrile block three-armed hydrophilic isonitrile
[0056] Add 15.2mg of the polymer prepared in Example 2 and 139.81mg (0.19mmol) of the three-armed hydrophilic isonitrile monomer into a 10mL polymerization bottle, vacuumize and inflate with nitrogen three times, add 0.8mL of dry tetrahydrofuran, and reflux at 55°C After 20 hours, 10 mL of methanol was added to quench the polymer, and the polymer was washed 5 times with methanol, centrifuged to obtain a white flocculent precipitate, and vacuum-dried until the mass remained unchanged.
[0057] The structural formula of the phenylisonitrile monomer in the present embodiment is:
[0058]
[0059] The structural formula of the product obtained is:
[0060]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com