Preparation method of secondary cross-linked resin adsorbent and secondary cross-linked resin adsorbent
A technology of secondary cross-linking and resin adsorption, applied in chemical instruments and methods, other chemical processes, alkali metal compounds, etc., can solve the problem that the secondary cross-linking method is not very thorough, the safety hazards are difficult to avoid residues, and the synthesis process is complicated. and other problems, to achieve the effect of good industrial application prospects, improved amphiphilicity, and simple synthesis process steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
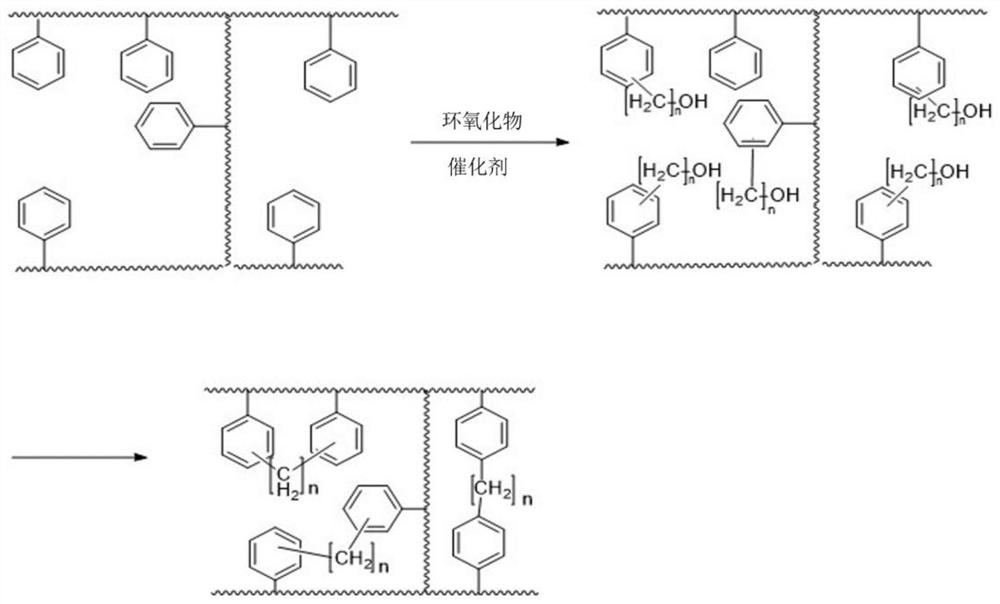
[0028] The preparation method of the secondary cross-linked resin adsorbent of the present invention, the synthesis process is as follows figure 1 As shown, after the monovinylbenzene suspension polymer beads are fully swollen in an organic solvent, under the action of a catalyst, they undergo a ring-opening addition reaction with an epoxide to introduce alcoholic hydroxyl groups or A "phenyl alkyl alcohol-catalyst" intermediate is generated, and the alcohol hydroxyl group or "alkyl alcohol-catalyst" will continue to undergo secondary cross-linking reaction with adjacent benzene rings to obtain a secondary cross-linked resin adsorbent. Wherein, adjacent refers to the adjacent or similar spatial position relationship, such as the front, back, left and right, up and down positions of the alcoholic hydroxyl group in three-dimensional space, as long as there are benzene rings in these positions, the alcoholic hydroxyl group will continue to cross-link with the benzene ring. The fi...
Embodiment 1
[0042] Add the premixed water phase into a 1L four-necked flask, and the composition of the water phase includes: 500 g of deionized water, 1 g of polyvinyl alcohol, and 20 g of sodium chloride. Stirring was started at room temperature, and the stirring speed was 100 rpm, so that the polyvinyl alcohol and sodium chloride were completely dissolved. Turn off the stirring and add the premixed oil phase. The composition of the oil phase includes: styrene 80g, methylstyrene 17g, divinylbenzene 3g, toluene 50g, 3# white oil 50g, azobisisobutyronitrile 0.3, peroxide Dibenzoyl 0.4g. Turn on the stirring to carry out the suspension polymerization reaction, the stirring speed is 120rpm, first raise the temperature to 65°C, react for 5h, then raise the temperature to 80°C and mature for 3h, to obtain the white balls of polymer beads. After the reaction is completed, the white balls of the polymer beads are filtered, washed with water, and washed with ethanol to become the precursor of t...
Embodiment 2
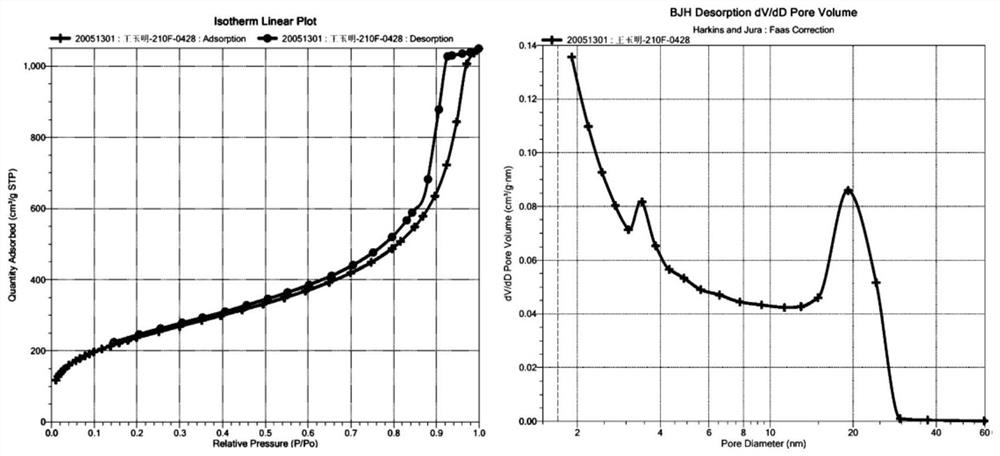
[0046] Add 75g of 1,2-dichloroethane and 10g of white polymer beads into a 500ml three-neck flask respectively, and let stand at room temperature to swell for 4h. After that, under mechanical stirring, add epichlorohydrin and anhydrous ferric chloride according to the ratio of (white ball: epichlorohydrin: anhydrous ferric chloride is 10:3:3 (weight ratio)), and control the stirring speed at room temperature 150rpm, react for 10 hours, then raise the temperature to 80°C and react for 10 hours. After the reaction was completed, when the temperature dropped to room temperature, the mother liquor was filtered out, stirred and washed with 60 g of acetone-hydrochloric acid solution at 130 rpm for 2 hours, then stirred and washed with 20 g of acetone for 3 times each time, and vacuum-dried at 60°C to constant weight to obtain a post-crosslinked resin Adsorbent. Polymer bead white ball raw material is the same as in Example 1, and the BET specific surface area of the secondary cro...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com