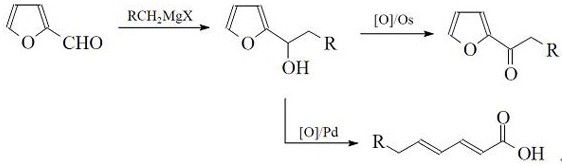
A kind of preparation method of gamma-substituted hexadienoic acid
A technology of hexadienoic acid and catalyst, applied in the preparation of carboxylate, the preparation of organic compounds, chemical instruments and methods, etc., can solve the problems of many side reactions, difficult separation, long process flow, etc., to reduce energy consumption and cost, reduce Consumption and emissions, the effect of reducing process flow
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] (1) Add 0.09mg of palladium chloride (0.0005mmol) into a 15ml pressure reaction tube, add 1.5ml of potassium dihydrogen phosphate buffer solution adjusted to pH 3 by phosphoric acid, add 1.0ml of tert-butanol and 0.1 mg of tetrabutyl chloride ammonium chloride, stirred evenly, replaced with 99% oxygen, then added 0.03 ml 1-(2-furyl)-1-methylmethanol (0.3 mmol), sealed the reaction tube, and heated in a water bath at 60°C Stir for 24 hours;
[0033] (2) Release gas after the reaction, cool the pressure reaction tube to room temperature, add 3 mL of ethyl acetate to the reaction solution for extraction, and carry out vacuum distillation on the upper layer of ethyl acetate to obtain 2,4-hexadienedioic acid (γ - position is hydrogen), and ethyl acetate is recovered, and analyzed by gas chromatography: 1-(2-furyl)-1-methylmethanol conversion rate is 100%, 2,4-hexadienedioic acid (γ-position is Hydrogen) yield greater than 92%.
Embodiment 2
[0035] (1) Add 4.5mg of palladium acetate (0.02mmol) into a 1L autoclave, add 300ml of sodium acetate buffer solution adjusted to pH 2.6 with hydrochloric acid, add 200ml of tert-butanol and 5mg of tetrabutylammonium chloride, and stir After uniformity, replace it with oxygen with a concentration of 98%, then add 10ml 1-(2-furyl)-1-methylmethanol (0.1mol), seal the high-pressure reactor, and react at a temperature of 60°C for 48 hours to control the oxygen pressure 0.3MPa;
[0036] (2) Release the gas after the reaction, cool the autoclave to room temperature, add 100ml ethyl acetate to the reaction solution and extract three times, combine the ethyl acetate phase, and carry out vacuum distillation on the ethyl acetate phase to obtain 2,4-hexyl acetate Dienoic acid (the γ-position is hydrogen), and ethyl acetate was recovered, and analyzed by gas chromatography: the conversion rate of 1-(2-furyl)-1-methylmethanol was 99.9%, and 2,4-hexadienoic acid ( The γ-position is hydroge...
Embodiment 3
[0038] 0.23mg of Pd(NO 3 ) 2 ·nH 2Add O (0.001mmol) into a 1L autoclave, add 300ml of sodium hydrogen phosphate buffer solution adjusted to pH 2.3 by phosphoric acid, add 100ml of ethyl acetate and 5mg of triphenylphosphine, stir well and use a concentration of 99% Then add 10ml of 1-(2-furyl)-1-ethylmethanol (0.1mol), seal the autoclave, and react at 80°C for 12 hours under temperature control, and control the oxygen pressure to 0.5MPa;
[0039] (2) Release the gas after the reaction, cool the autoclave to room temperature, add 100ml ethyl acetate to the reaction solution and extract three times, combine the ethyl acetate phase, and carry out vacuum distillation on the ethyl acetate phase to obtain 2,4-heptyl Dienoic acid (the γ-position is a methyl group), and ethyl acetate was recovered, and analyzed by gas chromatography: the conversion rate of 1-(2-furyl)-1-ethylmethanol was 98%, and the conversion rate of 2,4-heptadienoic acid (The γ-position is a methyl group) and th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com