Method for realizing N-N coupling of secondary arylamine by utilizing electrochemical reaction
A technology of chemical reaction and secondary aromatic amine, which is applied in the field of N-N coupling of secondary aromatic amine by electrochemical reaction, which can solve the problems of expensive catalyst, small substrate application range, unfriendly environment, etc., and achieve shortened reaction time and less restrictions , economical effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045]
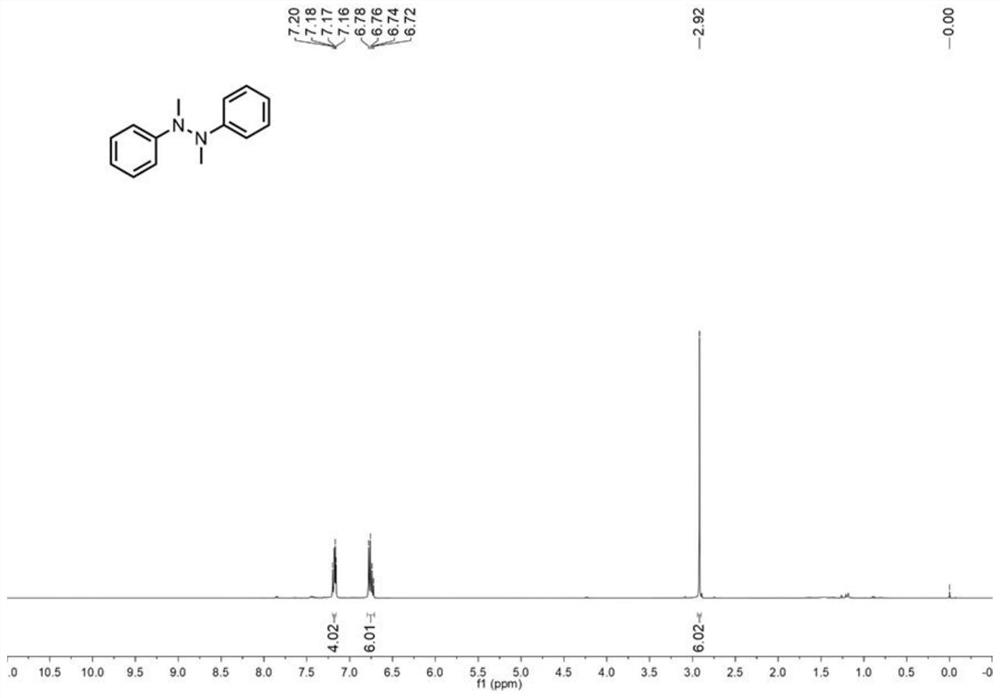
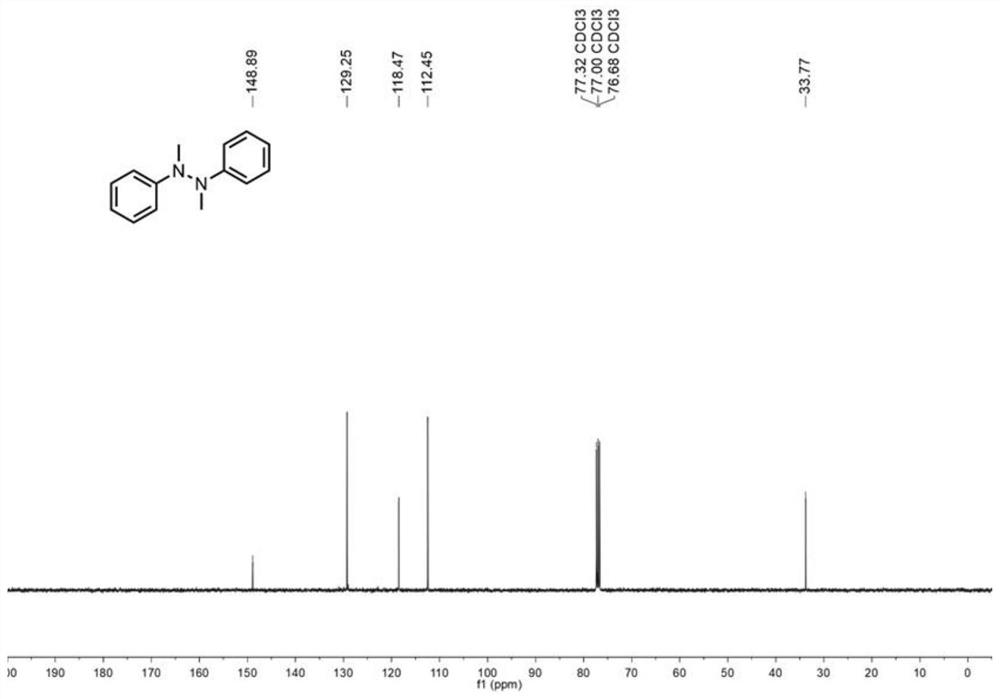
[0046] in such asFigure 8 Add 0.3mmol of N-methylaniline, 0.6mmol of sodium nitrite as electrolyte, 4.8mL of acetonitrile, 1.0mL of water and 1.2mL of ethanol in sequence to the 30mL electrolytic cell shown, and then put in a magnetic Stirrer, respectively add graphite carbon rod (Φ6mm), platinum sheet (1.0cm×1.0cm) as anode and cathode, turn on the power, adjust the current to 8.0mA, react at 450rpm, 50°C for 5 hours. After the reaction was completed, it was cooled to room temperature, the reaction solution was poured into 10 mL of water, extracted three times with ethyl acetate (10 mL), the organic phases were combined, and anhydrous Na 2 SO 4 Drying, concentration, separation by silica gel column chromatography (developing solvent: ethyl acetate / n-hexane=1 / 8), the target product was obtained, and the yield was 80% of N-N coupled tetrasubstituted hydrazine compounds. figure 1 and figure 2 shown. 1 HNMR (400MHz, CDCl 3 )δ7.20–7.16(m,4H),6.80–6.71(m,6H),2.92(...
Embodiment 2
[0048]
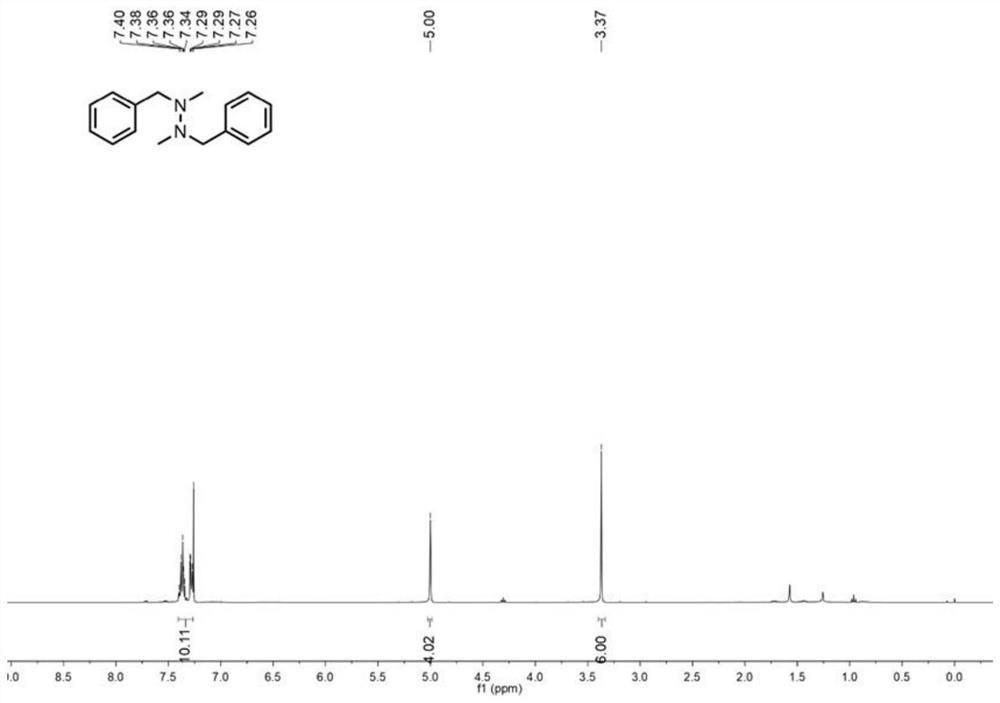
[0049] in such as Figure 8 Add 0.3mmol of N-methylbenzylamine, 0.6mmol of sodium nitrite as the electrolyte, 4.8mL of acetonitrile, 1.0mL of water and 1.2mL of ethanol in sequence to the 30mL electrolytic cell shown, and then add an appropriate size A magnetic stirrer was added with a graphite carbon rod (Φ6mm) and a platinum sheet (1.0cm×1.0cm) as the anode and cathode respectively. The power was turned on, the current was adjusted to 8.0mA, and the reaction was carried out at 450rpm and 50°C for 5 hours. After the reaction was completed, it was cooled to room temperature, the reaction solution was poured into 10 mL of water, extracted three times with ethyl acetate (10 mL), the organic phases were combined, and anhydrous Na 2 SO 4 Drying, concentration, silica gel chromatography column separation (developing solvent: ethyl acetate / n-hexane = 1 / 8) to obtain the target product, the yield of N-N coupled tetrasubstituted hydrazine compounds was 77%. image 3 and ...
Embodiment 3
[0051]
[0052] in such as Figure 8 Add 0.3mmol of diphenylamine, 0.6mmol of sodium nitrite as electrolyte, 4.8mL of acetonitrile, 1.0mL of water and 1.2mL of ethanol in sequence to the 30mL electrolytic cell shown, and then put in a magnetic stirrer of appropriate size, Add graphite carbon rods (Φ6mm) and platinum sheets (1.0cm×1.0cm) as anode and cathode respectively, turn on the power, adjust the current to 8.0mA, and react at 450rpm and 50°C for 5 hours. After the reaction was completed, it was cooled to room temperature, the reaction solution was poured into 10 mL of water, extracted three times with ethyl acetate (10 mL), the organic phases were combined, and anhydrous Na 2 SO 4 Drying, concentration, silica gel chromatography column separation (developing solvent: ethyl acetate / n-hexane = 1 / 8), to obtain the target product, the yield of 69% N-N coupled tetrasubstituted hydrazine compounds, NMR as shown in Figure 5 and Figure 6 shown. 1 HNMR (400MHz, CDCl 3 )δ7...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com