Near-infrared II thiapyran salt fluorescent compound capable of targeting mitochondria, preparation method and application thereof
A thiopylate and near-infrared technology, which is applied in the field of preparation of thiopylate fluorescent compounds, can solve the problems of difficult post-modification, poor bioavailability, complex synthesis process, etc., achieve good photostability, reduce energy gap, The effect of simple synthesis process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] The features and advantages of the present invention can be further understood through the following detailed description in conjunction with the accompanying drawings. The examples provided are only illustrative of the method of the present invention and do not limit the rest of the present disclosure in any way. Example 1] Synthesis and characterization of thiopyran compounds LQQ-1, LQQ-2, LQQ-3, LQQ-4 and LQQ-5
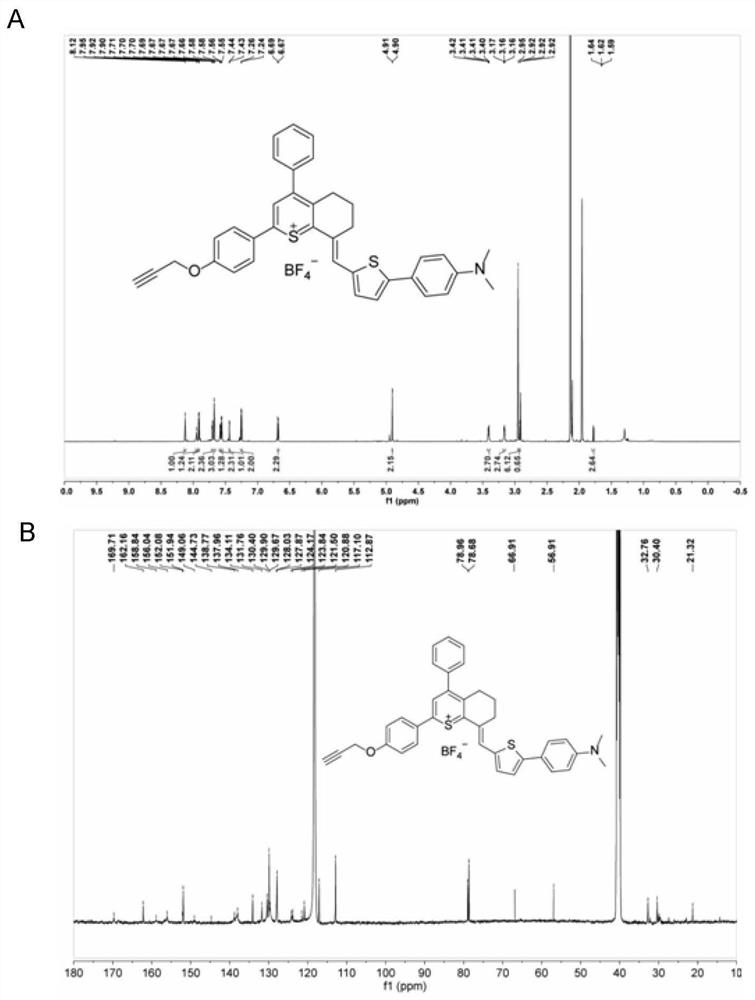
[0064] The thiopyran compounds LQQ-1, LQQ-2, LQQ-3, LQQ-4 and LQQ-5 (as shown below), all compounds were characterized by NMR and high-resolution mass spectrometry,
[0065]
[0066] Wherein, the synthesis route and steps of thiopyran compound LQQ-1 are as follows
[0067]
[0068] Synthesis of compound 3: KOH aqueous solution (50% w / w, 70 mL) was added to 4-hydroxyacetophenone 2 (100 mmol, 13.6 g) in 200 mL of MeOH in an ice bath. After stirring for 10 min, a solution of benzaldehyde 1 (100 mmol, 10.6 g) in 30 mL of MeOH was added dropwise over 1 h....
Embodiment 2
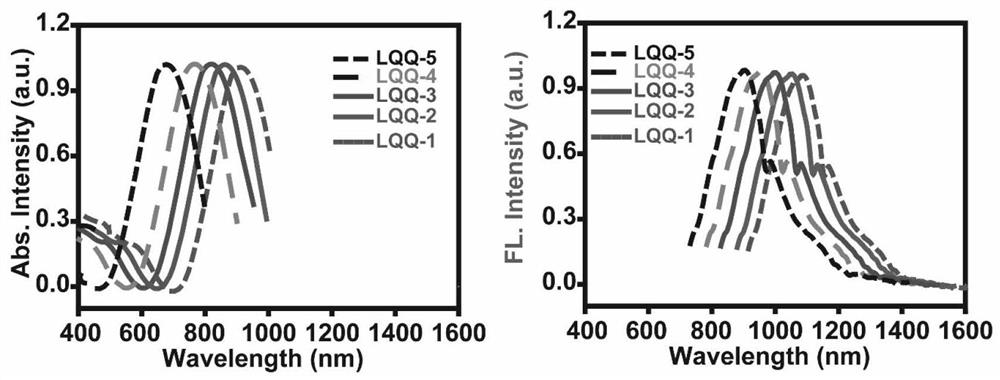
[0087] [Example 2] the ultraviolet absorption emission spectrum of dyestuff LQQ-1~LQQ-5
[0088] Weigh the dyes LQQ-1~LQQ-5 and dissolve them in DCM solvent at a concentration of 0.1~1mol / L, place them under a Shimadzu UV spectrometer to measure the absorption spectra of different dyes, and then perform normalized mapping. Then place the corresponding samples under a fluorescence spectrometer to measure their respective emission spectra, select 808nm as the excitation wavelength, and normalize their jinx respectively to obtain figure 2 , according to the spectrum, it can be seen that as more electron-donating groups are introduced into the molecule, the corresponding absorption-emission spectrum shifts bluer, and the more electron-withdrawing groups are introduced, the corresponding absorption-emission spectrum of the dye corresponds to redshift.
Embodiment 3
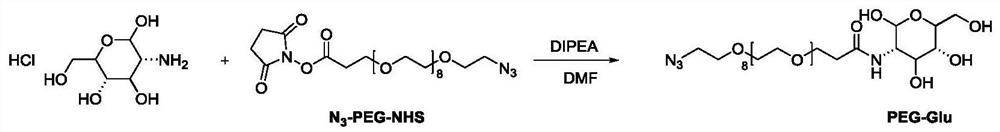
[0089] [Example 3] Preparation scheme of the near-infrared second region probe PEG-Glu
[0090]Weigh glucose hydrochloride (2.35mg, 0.01mmol) and N 3 -PEG 8 -NHS (6mg, 0.01mmol) was dissolved in 1mL of anhydrous DMF, then DIPEA (14mg, 0.1mmol) was added, and reacted overnight at room temperature. After the reaction was completed, the reaction solution was added to 50mL of ether and placed in a -80°C refrigerator The precipitate was precipitated, and then centrifuged to remove the upper ether clear liquid to obtain the crude product PEG-Glu, and the obtained crude product was directly put into the next step without purification. Its synthetic route is attached image 3 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com